Bacterial infections can be life-threatening if not treated quickly and appropriately. However, standard techniques for the diagnosis of bloodstream infections are time-consuming.
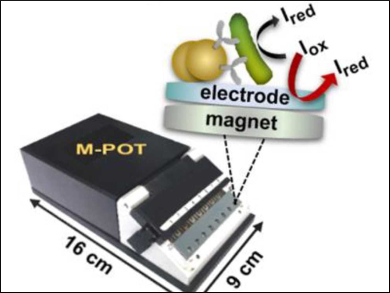
Hubert H. Girault, École Polytechnique Fédérale de Lausanne, Sion, Switzerland, and colleagues have developed an immuno-affinity amperometric method for the rapid diagnosis of bacterial infections in blood. Bacterial pathogens were isolated from human blood using magnetic beads coated with species-specific antibodies, e.g., anti-Escherichia coli. The captured bacteria were magnetically affixed to an electrode and then the reduction of certain redox indicators by the living bacteria was measured.
The bead‐captured bacteria were mixed with different redox indicators, e.g., 2,3,5‐triphenyl tetrazolium chloride (TTC), 2‐(4‐iodophenyl)‐3‐(4‐nitrophenyl)‐5‐(phenyl) tetrazolium chloride (INT), or 5‐cyano‐2,3‐di‐(p‐tolyl) tetrazolium chloride (CTC). The redox states of the indicators were then monitored amperometrically, using a multiplexed potentiostat (M-POT, pictured). The measured current provides information about the redox state of the indicators and, by proxy, about the concentration of the targeted bacteria in the blood sample.
The method provides a reliable diagnosis within several hours, which is much faster than the multiday methods currently used in hospitals. The researchers believe the technology could allow the early detection of pathogens and the fast delivery of an appropriate anti-infective treatment.
- Immuno-affinity Amperometric Detection of Bacterial Infections,
Yingdi Zhu, Milica Jović, Andreas Lesch, Lysiane Tissières Lovey, Michel Prudent, Horst Pick, Hubert H. Girault,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201808666