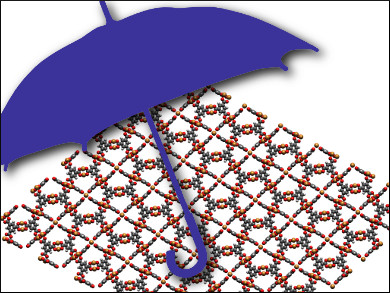
Metal–organic frameworks (MOFs) have many possible applications: as molecular sieves or catalysts, for energy conversion or drug delivery, as gas storage materials or sensors. They combine many of the benefits of porous minerals such as zeolites with a designer nature that allows them to be tailored very precisely to specific applications. Unfortunately, many MOFs fall apart quite readily—they crumble, crumple, and collapse, especially when water is present. Given the almost ubiquitous nature of water, this is a major hindrance.
“Sacrificial” Bonds Provide Hydrolytic Stability
Russell E. Morris, University of St. Andrews, UK, and Charles University in Prague, Czech Republic, and colleagues have developed a concept with the potential to endow a variety of MOFs with hydrolytic stability. The concept involves so-called “crumple zones” for MOFs, which feature sacrificial bonds that can be broken when the MOF is under stress. These sacrificial bonds are broken instead of bonds that are essential for structural integrity, so the overall system does not collapse. The concept is akin to the energy-absorbing crumple zones built into cars and other vehicles that take the stress of an impact and are designed to reduce the amount of energy that hits driver and passengers.
MOFs are usually constructed from inorganic building units—metal ions or clusters—that are linked together using multitopic organic ligands to form three-dimensional, crystalline materials. Many of these, but not all, are highly porous and have an enormous internal-surface-area-to-volume ratio. This characteristic is used in heterogeneous catalysis and in other areas of materials science. Thousands of new MOFs are constructed each year and the total reported over the last two decades or so now stands at around 20,000.
Hydration instead of Disintegration
The researchers have prepared an analog of the MOF known as HKUST-1. The new material, called STAM-17-OEt, is composed of copper “paddlewheel” clusters. The team has added sacrificial bonds in the coordination environment of its metal centers, which they refer to as hemilability. This gives the dehydrated copper-based MOF much greater hydrolytic stability.
When the dehydrated MOF is exposed to water, the team found, there is no indiscriminate breaking of the coordination bonds that hold the framework together. Instead, it is the non-structural weak interactions between the copper paddlewheel clusters that are broken in the presence of water and the overall framework itself recovers the original as-synthesized, hydrated structure. In contrast, HKUST-1, without the sacrificial bonds, simply disintegrates within a day.
The presence of the sacrificial bonds in STAM-17-OEt, which are lost as water is taken on, does not disrupt the designed structure nor the functionality of the MOF. Rather their loss is essentially the final step in the maturation of the synthesized MOF to its active, usable, and stable state.
Preserving Structural Integrity
At the fundamental level, the team explains their concept as follows: “Our hypothesis is that if the material is hemilabile and allows the formation of new weak bonds upon removal of the coordinated water molecules, then as water is readsorbed into the material, energy released by the metal–water adsorption interaction is used to preferentially break the weak bonds in the structure, leaving the structural integrity of the metal-bridging ligand bonds intact.”
“The next stage is to further develop the applications of materials of this type. In collaboration with the Defence Science and Technology Laboratory, the UK’s military research institution, the team is developing toxic gas adsorption applications of STAM-17-OEt for use in personal protection—something that would not be possible without the enhanced stability shown by the material,” Morris told ChemViews Magazine.
- Hydrolytic stability in hemilabile metal–organic frameworks,
Lauren N. McHugh, Matthew J. McPherson, Laura J. McCormick, Samuel A. Morris, Paul S. Wheatley, Simon J. Teat, David McKay, Daniel M. Dawson, Charlotte E. F. Sansome, Sharon E. Ashbrook, Corinne A. Stone, Martin W. Smith, Russell E. Morris,
Nat. Chem. 2018, 10, 1096–1102.
https://doi.org/10.1038/s41557-018-0104-x