What a surprise. Johan Winne, Richard Hoogenboom and their teams at Ghent University, Belgium, planned the investigation of polypeptide-like macromolecules, but ended up with the first synthesis and characterization of a seven-membered cyclic imino ether [1]. The unexpected shift in their research focus came when the scientists realized the published oxazepine structure was not what it was supposed to be.
From Oxazolines to Oxazepines
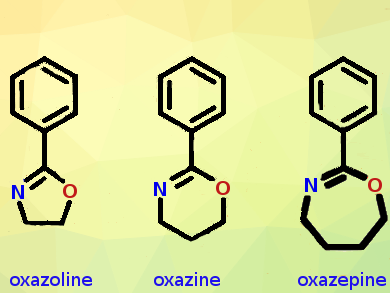
Oxazepines are two-carbons-expanded versions of the oxazoles, five-membered heterocycles with nitrogen and oxygen replacing two carbon atoms. Oxazolines are hydrogenated oxazoles, and 1,3-oxazolines can be regarded as cyclic imino ethers. 2-Substituted 1,3-oxazolines are extensively explored as they can polymerize to poly(2-oxazoline)s in a process called living cationic ring-opening polymerization. The resulting polymers resemble polypeptides in that they are water-soluble, have an amide function in every repeating unit, and their side chains and end groups can be endowed with additional functionality and architecture. These properties make the poly(2-oxazolines) very interesting materials, especially for biomedical applications.
Besides side-chains, end groups, and the polymerization process, the polymer backbone in the polymeric cyclic imino ethers could be varied as well. To make the backbone longer or shorter, the ring size of the monomer must be altered. Five-membered oxazolines give a two-carbon backbone in the polymer repeating unit, and six-membered 1,3-oxazines form polymers with three-carbon backbones. A longer backbone would make significant differences in performance.
To extend the backbone size even further, to four carbon atoms, the Ghent groups first had to synthesize the monomer, a seven-membered fully dehydrogenated phenyl-substituted 1,3-oxazepine. However, its synthesis turned out not to be straightforward. Literature data on the compound were scarce, the researchers said, and following one of the published protocols, the researchers were surprised to find a benzoylated pyrrolidine, and not the intended oxazepine.
“It has in fact never been prepared.”
Spectra comparing the synthesized substance with benzoylated pyrrolidine confirmed that the published syntheses yielded different molecules. “This led us to the remarkable conclusion that the parent heterocyclic structure … has in fact never been prepared,” reported the scientists. The seven-membered cyclic imino ether was still elusive.
Therefore, the scientists developed a different strategy and eventually synthesized the elusive oxazepine in two steps and in reasonable yield. Its correct structure was confirmed by its X-ray diffraction pattern.
Oxazepine Polymers?
Cationic ring-opening polymerization, however, turned out to be a sluggish business, the researchers reported. After days of reaction, merely oligomers were found in the product spectrum. To explain this outcome, they referred to the reaction mechanism.
In living cationic ring-opening polymerization, not the release of ring strain pushes the reaction forward, but the amide group evolving from isomerization of the positively charged imino ether is thermodynamically more stable. Therefore, the kinetics of the reaction depends on the accessibility of the tertiary amide.
When expanding the ring size, stereoelectronic effects become dominant. For the seven-membered ring, the researchers observed a “significantly deplanarized” environment around the imino ether oxygen, and efficient resonance with the electron-withdrawing imino nitrogen is no longer possible. A planar cationic transition state can only be attained when additional ring strain is imposed, the researchers argue. They concluded that this nonplanar ring configuration would also explain why the previous attempts to form the seven-membered oxazepine ring have failed in the first place.
Although the intended analysis of a new polymer with a longer backbone was not realized, this work illustrates once more the possible twists and turns in experimental science. Even the best analytic tools cannot prevent assignment errors, and many more structures may bear surprises. For the newly found member of the class of cyclic imino ethers, however, the researchers propose further explorations as an asymmetric ligand and for drug development.
- The Elusive Seven-Membered Cyclic Imino Ether Tetrahydrooxazepine,
Bart Verbraeken, Jan Hullaert, Joachim van Guyse, Kristof Van Hecke, Johan Winne, Richard Hoogenboom,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b10918