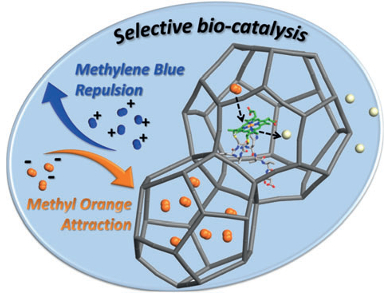
Enzymes are biological entities with high catalytic activities relevant for many industrial processes. Their stabilization and protection are, therefore, important. Enzymes can be stabilized by immobilization within a solid host support—e.g., in metal–organic frameworks (MOFs), a class of porous materials.
Clémence Sicard, Institut Lavoisier de Versailles, Université Paris-Saclay, France, Rémy Ricoux, Institut de Chimie Moléculaire et des Matériaux d’Orsay, Université Paris Sud, Université Paris-Saclay, France, and colleagues have used nanoparticles of the stable MOF MIL-101(Cr) (MIL = Matériaux Institut Lavoisier) to encapsulate the enzyme microperoxidase-8 (MP8). MP8 is a minienzyme that has remarkable peroxidase and monooxygenase activities, but can be deactivated under acidic conditions and lacks high selectivity toward reactants.
The encapsulation of MP8 in MIL-101(Cr) significantly improved its catalytic activity under acidic conditions. It also greatly enhanced its selectivity for the degradation of the harmful negatively charged organic dye methyl orange. This results from the selective adsorption of the dye on the MIL-101(Cr) support through electrostatic interactions. The results demonstrate the effectiveness of MOFs as enzyme immobilization materials. They also show the role of MOFs as active components that can induce selectivity in the catalytic process.
- Enzyme Encapsulation in Mesoporous Metal-Organic Frameworks for Selective Biodegradation of Harmful Dye Molecules,
Effrosyni Gkaniatsou, Clémence Sicard, Rémy Ricoux, Linda Benahmed, Flavien Bourdreux, Qi Zhang, Christian Serre, Jean-Pierre Mahy, Nathalie Steunou,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201811327