New Cancer-Treatment Approaches
Some severe forms of leukemia develop because proteins on the epigenetic level lose their regulative function. In a broad international collaboration, Paul E. Brennan, Oleg Fedorov, University of Oxford, UK, and colleagues have identified molecules that can effectively inhibit the dysregulated proteins. The team discovered and designed potential drugs and tested them on the cellular level. The findings set the stage for new biological experiments and cancer-treatment approaches in the future.
To regulate gene expression, cells use chemical signals to mark DNA and the histones around which the DNA is wrapped. This control of gene expression is called epigenetics, and disorders in epigenetic regulation have been found to be crucial for certain types of cancer development.
For example, acute myeloid leukemia—a severe cancer type in which myeloid blood cells grow rapidly and abnormally and accumulate in the bone marrow and blood—develops when proteins that read epigenetic marks become seriously dysregulated. The reader proteins recognize modified lysine residues, an amino acid that is part of the histone tails, and trigger leukemia-driving programs of gene expression.
Inhibiting Epigenetic Factors
However, the dysregulated proteins may also be treatable. Drugs binding specifically to these proteins, especially their lysine-binding protein domains, could prevent the onset of leukemia on the epigenetic level. These drugs—small molecules mimicking the modified lysines—are targeted by the team. To identify possible candidates, the scientists first screened a library of chemical compounds for their ability to bind to the modified-lysine-binding domains—the so-called YEATS domains.
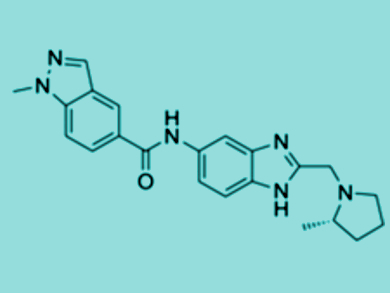
The screening resulted in one initial hit, which was a small molecule with a core benzimidazole motif. Benzimidazoles are well-established drugs that are comprised of fused benzene and nitrogen-containing aromatic rings.
Selective Chemical Probes
Starting with the identified benzimidazole derivative, the researchers systematically altered the structure to improve binding and make the molecule more stable. After vigorously testing a set of two hundred molecules, they ended up with a potent and stable molecular inhibitor (pictured), which bound two proteins containing the YEATS domains very tightly. In various assays, the team tested its activity toward the dysregulated proteins and characterized the specific YEATS binding mode.
The researchers pointed out that their discovery was the first report on a selective and powerful inhibitor of epigenetic factors promoting acute myeloid leukemia. This cancer progresses rapidly and is typically fatal within weeks or months if left untreated. Alternatives to chemotherapy remain scarce and new treatment approaches are desperately sought. It was further emphasized that this detailed knowledge of the binding mode will be a promising starting point for further drug development in cancer therapy.
- Discovery of an MLLT1/3 YEATS Domain Chemical Probe,
Moses Moustakim, Thomas Christott, Octovia P. Monteiro, James Bennett, Charline Giroud, Jennifer Ward, Catherine M. Rogers, Paul Smith, Ioanna Panagakou, Laura Díaz-Sáez, Suet Ling Felce, Vicki Gamble, Carina Gileadi, Nadia Halidi, David Heidenreich, Apirat Chaikuad, Stefan Knapp, Kilian V. M. Huber, Gillian Farnie, Jag Heer, Nenad Manevski, Gennady Poda, Rima Al-awar, Darren J. Dixon, Paul E. Brennan, Oleg Fedorov,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201810617