In molecular electronics, individual molecules are used as electronic components. π-Conjugated systems, for example, can be regarded as molecular wires. The development of molecular wires with high conductance is an interesting research target.
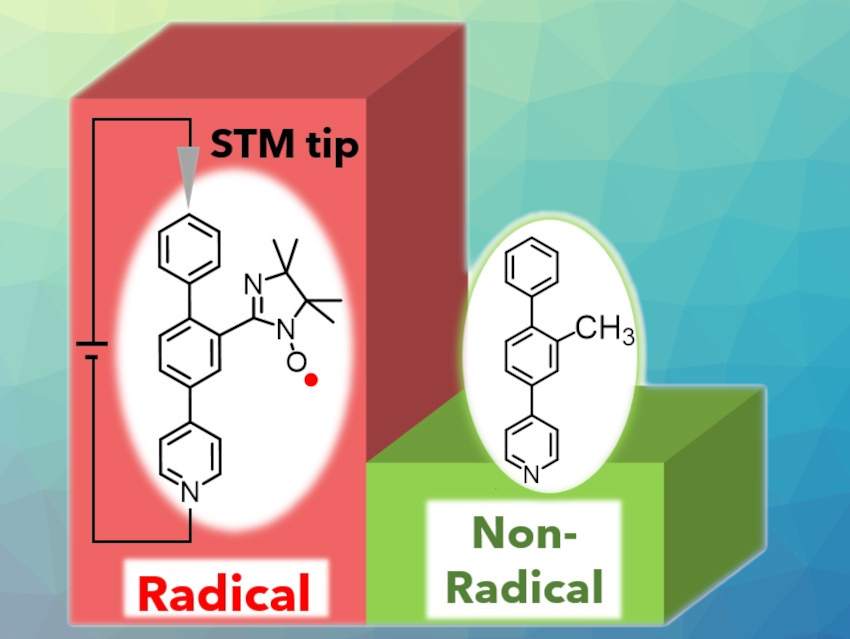
Ryuto Yasui, Daiki Shimizu, and Kenji Matsuda, Kyoto University, Japan, investigated whether a radical substituent enhances the conductance of a molecular wire. The team synthesized two molecular wires with and without a radical substituent (pictured) and subjected them to STM measurements. The STM technique measures tunneling current passing through single molecules so that the conductance of a molecule can be evaluated. The molecular wires are 4-(biphenyl-4-yl)pyridine derivatives, which were attached onto metalloporphyrins to anchor them to the substrate.
A statistical analysis of the STM measurements showed that the radical-substituted wire has a 3.2 times larger conductance than the non-radical-substituted reference wire. Quantum chemical calculations suggest that an energetically high-lying SOMO (singly occupied molecular orbital) originating from the radical substituent is likely the origin of the effect, even though only 17 % of this SOMO is distributed along the wire. This shows that even the partial delocalization of singly occupied orbital can strongly alter the conductance of a molecular wire.
- Large Enhancement of Single Molecular Conductance of Molecular Wire by a Radical Substituent,
Ryuto Yasui, Daiki Shimizu, Kenji Matsuda,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202104242