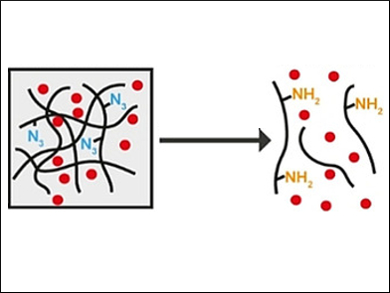
Stimuli-responsive hydrogels are promising materials for drug delivery. The hydrogels can be designed to encapsulate a drug as cargo. A specific stimulus triggers a transition from a gel to a solution, which releases the drug.
Allan Gamble and colleagues, University of Otago, New Zealand, have developed a way to induce hydrogel dissolution via a bio-orthogonal bond-cleavage reaction. A dipeptide hydrogel with azide capping groups (p‐azidobenzyl carbamate‐PhePhe dipeptide) was used to encapsulate the anticancer drug doxorubicin (pictured in red).
The addition of a strained alkene, trans‐cyclooctene (TCO), results in the gel-to-solution transition and the release of doxorubicin (pictured below). This is caused by an alkene–azide 1,3‐dipolar cycloaddition between the TCO and the azide, followed by hydrolysis and conversion to an amine (pictured in yellow).
.jpg)
This “click-to-dissolve” strategy enables the release of the hydrogel cargo. It complements the current arsenal of stimuli-responsive hydrogels that are available. Further modifications to the peptide and the azide-capped linker could lead to alkene-induced gel dissolution at varying rates and broaden the range of applications.
- Alkene-Azide 1,3-Dipolar Cycloaddition as a Trigger for Ultrashort Peptide Hydrogel Dissolution,
Sumit Dadhwal, Jessica M. Fairhall, Shailesh K. Goswami, Sarah Hook, Allan B. Gamble,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201801184