Tyrosinases and catechol oxidases are coupled binuclear copper (CBC) enzymes. These enzymes catalyze the oxidation of phenolic compounds. CBC enzymes play an important physiological role in protecting cells from chemical damage. They also have potential applications in multiple industrial processes, which makes it important to understand their reaction mechanisms.
Tyrosinases can monooxygenate phenolic substrates to o-diphenols (monophenolase activity) and oxidize them further to o-diquinones (diphenolase activity). Catechol oxidases are believed to have only diphenolase activity.
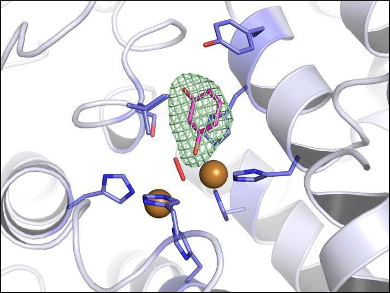
Nina Hakulinen and colleagues, University of Eastern Finland, Joensuu, have studied the structure and function of a catechol oxidase (AoCO4) from the fungus Aspergillus oryzae. To understand the structure, the team determined the crystal structure of AoCO4 bound to resorcinol (1,2-benzenediol) at a 2.2 Å resolution (pictured). In addition, the reaction products of the AoCO4-catalyzed oxidation of resorcinol, phenol, and of all isomers of di- and triphenols were characterized using mass spectrometry.
Surprisingly, AoCO4 has a monooxygenase activity towards small phenolic compounds. The binding mode of resorcinol in the crystal structure is a nonproductive, i.e., non-reactive, binding mode. However, this unproductive binding mode competes with a second, reactive binding mode. Such an additional reactivity–besides the main reaction–is called enzyme promiscuity. According to the researchers, the results provide an improved basis for the discussion of differences in the catalytic activity between tyrosinases and catechol oxidases.
- Unraveling Substrate Specificity and Catalytic Promiscuity of Aspergillus oryzae Catechol Oxidase,
Leena Penttinen, Chiara Rutanen, Janne Jänis, Juha Rouvinen, Nina Hakulinen,
ChemBioChem 2018, 19, 2348–2352.
https://doi.org/10.1002/cbic.201800387