Metal Nanoparticles of Uniform Size and Shape
Finding a way to synthesize small metal-containing nanoparticles consistently could revolutionize heterogeneous catalysis, especially if those nanoparticles were highly active in water. Josiel Barbosa Domingos, Federal University of Santa Catarina, Florianopolis, Brazil, and colleagues have devised a new approach to the synthesis of palladium(II) iodide nanoparticles.
The team has demonstrated the efficacy of these nanoparticles as catalysts in an aryl alkenylation reaction with water as the solvent. The work might address some of the problems facing nanoscience in the quest to develop a “reliable, simple and environmentally friendly method” to synthesize metal nanoparticles of both uniform size and shape.
Overcoming Analytical Limitations
The team points out that one of the limiting factors has been the lack of analytical techniques capable of investigating metal nanoparticles and the processes used to make them in the requisite detail. They add that, “Synchrotron-based techniques provide a better resolution and the possibility of conducting time-based experiments.”
The researchers used ligand exchange in the presence of poly(vinyl pyrrolidone) in an aqueous medium at room temperature to synthesize small, spherical nanoparticles of palladium iodide. They were able to follow the formation and investigate the product itself using in situ time-resolved synchrotron-based small-angle X-ray scattering (SAXS) and X-ray absorption near edge structure and extended X-ray absorption fine structure (XANES/EXAFS) analysis. These techniques can probe structure in ways that conventional X-ray diffraction and crystallographic techniques cannot.
Dual Catalytic Functionality
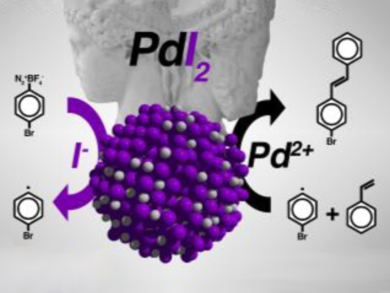
The resulting palladium(II) iodide nanoparticles have a dual catalytic functionality (pictured). In an aryl alkenylation reaction, they work in the absence of base or ligand in an aqueous medium. The experimental evidence suggests that the catalytic process follows a single-electron transfer (SET) pathway mechanism, wherein the iodide acts as a radical promoter and the palladium metal atoms are an activator of the alkene moiety. “This new protocol could evolve into a broadly applicable radical reaction for the functionalization of alkenes,” the team suggests. Critically, the team showed that their palladium(II) iodide nanoparticles performed better than the conventional catalyst.
The team is now screening various diazonium salts and other olefins under the optimized reaction conditions they have developed to see whether their nanocatalyst might be useful for a wider range of important industrial chemical processes. “We are now checking the scope of this reaction for this catalyst and if the proposed mechanism holds for structurally diverse substrates,” Domingos told ChemViews Magazine.
- On the Formation of Palladium(II) Iodide Nanoparticles: An In Situ SAXS/XAS Study and Catalytic Evaluation on an Aryl Alkenylation Reaction in Water Medium,
Josiel Barbosa Domingos, Eloah Latocheski, Marcelo V. Marques, Brunno L. Albuquerque, Thalia J. Schuh, Aline M. Signori, Daniela C. Oliveira, Taransankar Pal,
ChemCatChem 2018.
https://doi.org/10.1002/cctc.201801817