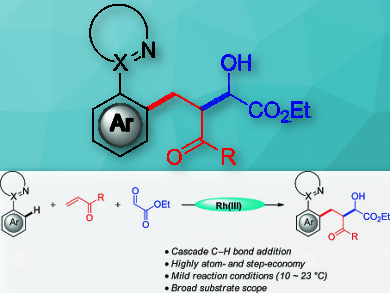
The catalytic activation of otherwise inert C–H bonds has attracted much attention in molecular synthesis in recent years. Transition‐metal‐catalyzed C–H alkylation is a powerful tool for eco‐friendly C–C forming reactions.
Lutz Ackermann, Georg‐August‐Universität Göttingen, Germany, Jie Li, Jiangnan University, Wuxi, Jiangsu, China, and colleagues have developed a straightforward synthesis of β‐acyl substituted arylbutanoates through a rhodium(III)‐catalyzed three‐component C–H alkylation/nucleophilic addition (pictured). Various synthetically useful aryl oximes and pyrazoles can be reacted under mild reaction conditions.
The team obtained valuable β‐acyl substituted arylbutanoates in moderate to good yields. The strategy shows excellent atom‐ and step‐economy, high chemo‐ and site‐selectivity, a broad substrate scope, and a wide functional group tolerance. Preliminary mechanistic studies support a rate‐determining base-assisted intramolecular electrophilic substitution-type (BIES)‐type C–H activation. It preferentially occurs at electron‐rich arenes.
- Rhodium(III)‐Catalyzed C–H Alkylation/Nucleophilic Addition Domino Reaction,
Mengyao Tang, Yunpeng Li, Shengnan Han, Lei Liu, Lutz Ackermann, Jie Li,
Eur. J. Org. Chem. 2018.
https://doi.org/10.1002/ejoc.201801535