Cytochrome P450 enzymes (P450s) catalyze various reactions, transforming an extraordinarily broad range of substrates in a regio- and stereospecific manner. The best-known reaction of these enzymes is the hydroxylation of inert C–H bonds, which is synthetically challenging. As such, P450s are promising for chemical synthesis. However, P450s that catalyze both aliphatic and aromatic hydroxylation are rare.
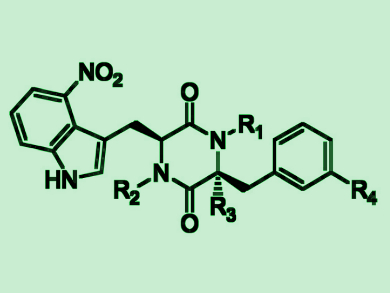
Yousong Ding, University of Florida, Gainesville, USA, and colleagues have found that one P450, called TxtC, can sequentially catalyze aliphatic and aromatic hydroxylations in the biosynthesis of the bacterial natural products thaxtomins (pictured), which have a diketopiperazine (DKP) core. Coupled with two other thaxtomin biosynthetic enzymes, the team used TxtC for the synthesis of over 40 hydroxylated thaxtomin-like compounds. This demonstrates the big substrate promiscuity of the enzyme.
The researchers further synthesized several hydroxylated thaxtomin-unlike compounds by combining the engineered TxtC with enzymes from other bacteria and an engineered human enzyme. According to the team, their work could pave the way for future engineering of TxtC for broader synthetic applications.
- A Promiscuous Cytochrome P450 Hydroxylates Aliphatic and Aromatic C-H Bonds of Aromatic 2,5-Diketopiperazines,
Guangde Jiang, Yi Zhang, Magan M. Powell, Sarah M. Hylton, Nicholas W. Hiller, Rosemary Loria, Yousong Ding,
ChemBioChem 2019.
https://doi.org/10.1002/cbic.201800736