Zintl phases and Zintl ions have been thoroughly investigated in both solid-state and molecular chemistry for decades. Particularly the polypnictides, based on P, As, Sb, or Bi, feature a rich chemistry of chain- and cage-type compounds, with the polycyclic [P7]3– anion as one of the most prominent examples.
When it comes to complexes of such ions, previous research has been almost entirely focused on transition metals and phosphorus. One reason is the availability of the molecular and soluble phosphorus allotrope P4. In contrast, yellow arsenic, As4, is inconvenient to synthesize, extremely photosensitive, and highly prone to decomposition.
Peter W. Roesky and colleagues, Karlsruhe Institute of Technology (KIT), Germany, have combined organometallic f-element chemistry and nanochemistry to generate f-element polyarsenides from elemental arsenic. First, the team synthesized elemental As0 nanoparticles (7.2±1.8 nm in diameter) as reactive arsenic source. This was achieved by combining a suspension of AsI3 in tetrahydrofuran (THF) with a solution of lithium naphthalenide at 0 °C.
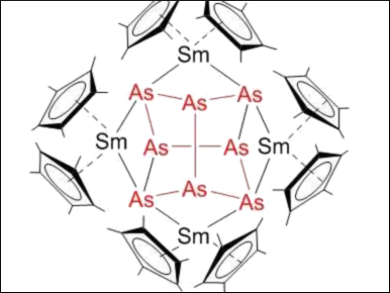
By reacting [Cp*2Sm] (Cp* = η5-C5Me5) as a reductive f-element compound with these As0 nanoparticles, the samarium polyarsenide complexes [(Cp*2Sm)2(μ-η2:η2-As2)] and [(Cp*2Sm)4As8] were obtained. This approach avoids the use of As4. [(Cp*2Sm)4As8] (pictured) is the largest molecular polyarsenide of the f-elements.
- Samarium Polyarsenides Derived from Nanoscale Arsenic,
Peter Werner Roesky, Christoph Schoo, Sebastian Bestgen, Alexander Egeberg, Jasmin Seibert, Sergey N Konchenko, Claus Feldmann,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201813370