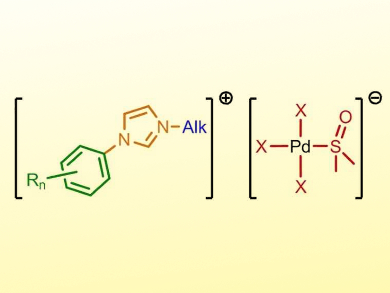
Ionic Liquids—salts with a melting point below 100 °C—can be modified in various ways. The so-called tunable aryl alkyl ionic liquids (TAAILs) are based on a cationic imidazolium core with different N-aryl rings and N-alkyl chains, as well as a counter-anion, all of which can be independently varied.
Thomas Strassner and colleagues, Technical University Dresden, Germany, have synthesized a range of TAAILs whose anion contains palladium atoms (example pictured). This type of metal-containing ionic liquid is readily available by dissolving a palladium chloride or bromide and the desired imidazolium salt in organic solvents such as dimethylsulfoxide (DMSO). This synthetic route tolerates a variety of reagent combinations.
Many of the synthesized salts are liquid at room temperature; others possess melting points well below 100 °C. The team verified the molecular structure of the ionic liquids using different analytical methods, including X-ray diffraction of their solid forms. The combination of the dissolution and physical properties of ionic liquids with the catalytic activity of palladium complexes could be very useful.
- Tailored Palladate Tunable Aryl Alkyl Ionic Liquids (TAAILs),
Felix Schroeter, Johannes Soellner, Thomas Strassner,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201804431