The well-known cyclobutane molecule (C4H8) contains four carbon atoms connected by four two-center two-electron (2c,2e) bonds. It has a total of eight skeletal electrons. The compound becomes electron-deficient when electrons are removed from the four bonds. If several electrons are removed, the covalent bond energy no longer compensates the electrostatic repulsion in the cation and the compound decomposes.
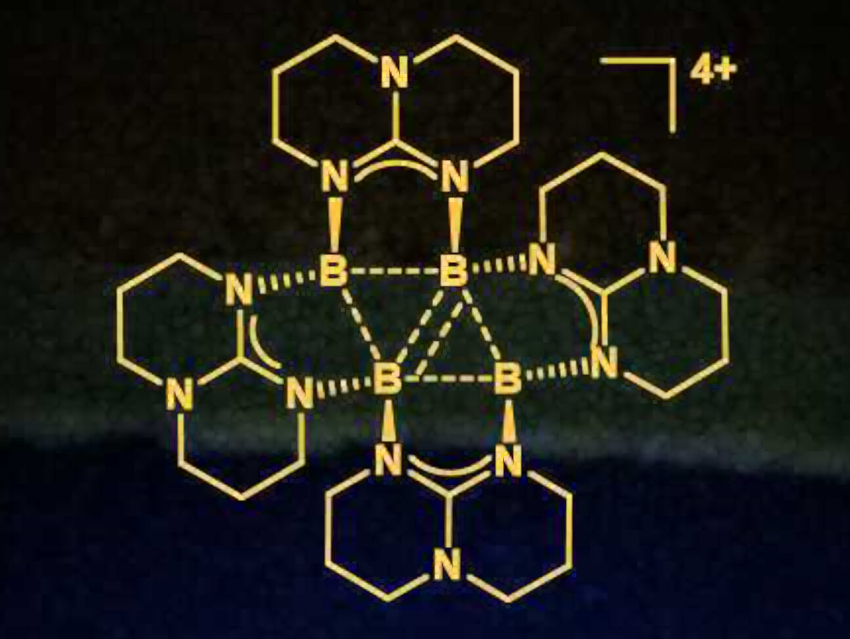
Hans-Jörg Himmel and colleagues, University of Heidelberg, Germany, have synthesized an unprecedented base-stabilized σ‐aromatic tetraborane(4) tetracation. The compound (pictured) was prepared from the dihydridodiborane [HB(hpp)]2 (hpp=1,3,4,6,7,8‐hexahydro‐2H‐pyrimido[1,2‐a]pyrimidinate) by hydride abstraction.
The product has a rhomboid-type B4 skeleton and only four skeletal electrons, i.e., it can be considered a [B4H4]4+ analogue. It is isoelectronic with the unstable cyclobutane tetracation, yet is stable itself. Within the structure, electrostatic repulsion is reduced by delocalization of the high positive charge into four bicyclic guanidinate groups.
The bright-orange salts of the new σ-aromatic tetraborane are fluorescent, with a maximum fluorescence signal at 580 nm. The researchers believe such electron-deficient rings could be integrated into larger molecular architectures for use in optoelectronic applications.
- Four Boron Atoms, Four Positive Charges, and Four Skeletal Electrons: A Fluorescent σ-Aromatic Tetraborane(4),
Anna Widera, Hubert Wadepohl, Hans-Jörg Himmel,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201814041