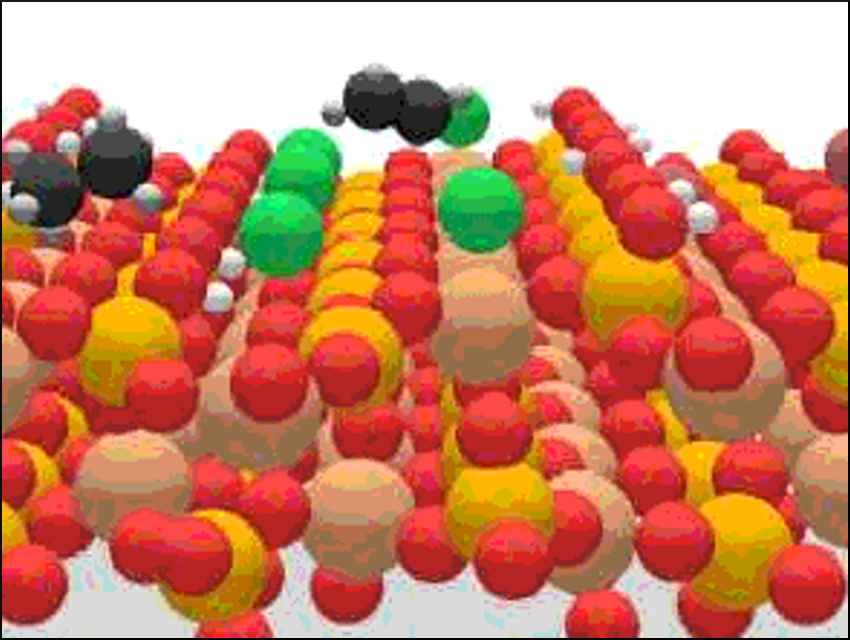
Alkane functionalization is a hurdle for the development of efficient processes in natural gas upgrading. Catalytic alkane oxyhalogenation is a promising route for the one-step production of olefins. The selectivity of this alkane activation is steered by the halogen used.
Javier Pérez‐Ramírez, ETH Zurich, Switzerland, and colleagues have investigated the mechanistic origin of this effect. The team discovered that chlorine drives the selective conversion of alkanes to olefins as a result of surface confinement. Bromine use leads to a gas-phase radical mechanism that produces alkyl bromide, cracking, and combustion products.
The team used a combination of advanced operando techniques, including prompt gamma activation analysis (PGAA) and photoelectron photoion coincidence spectroscopy (PEPICO). PGAA was used to probe processes at the catalyst surface and PEPICO for phenoma in the gas phase (pictured below). In PGAA, non-destructive cold prompt neutrons bombard the nuclei of atoms and produce prompt γ-rays that are emitted with element-specific energies and intensities. PEPICO involves photoionization by monochromatic vacuum ultraviolet (VUV) synchrotron radiation, which leads to the splitting of excited molecules into ions and electrons.
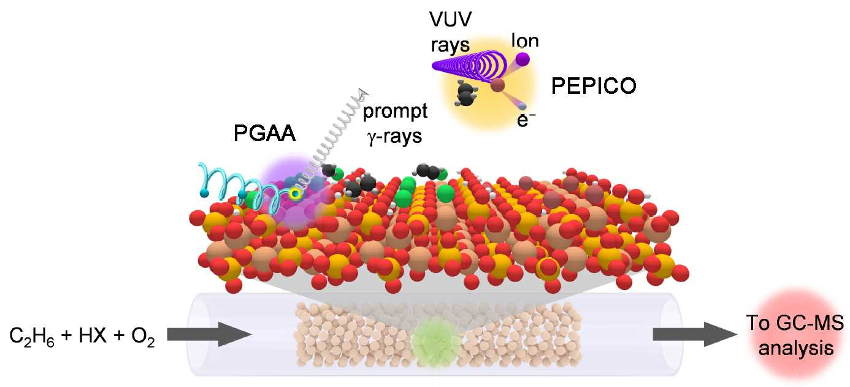
The results demonstrate that chlorine‐based processes have great potential for one‐step olefin production on a technical scale. They also provide guidelines for the development of catalysts for direct alkane‐to‐olefin transformations via oxyhalogenation. The researchers believe the strategy can be applied to other hydrocarbon functionalization processes.
- Halogen-Dependent Surface Confinement Governs Selective Alkane Functionalization to Olefins,
Guido Zichittella, Matthias Scharfe, Begoña Puértolas, Vladimir Paunović, Patrick Hemberger, Andras Bodi, László Szentmiklósi, Núria López, Javier Pérez‐Ramírez,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201811669