Carbon dioxide (CO2) is a harmful greenhouse gas. Chemists could help to find ways to reduce CO2 levels in the atmosphere. However, it costs a lot of energy to break the molecule’s C=O bonds and convert CO2 back to one of its original energy-rich reduced forms.
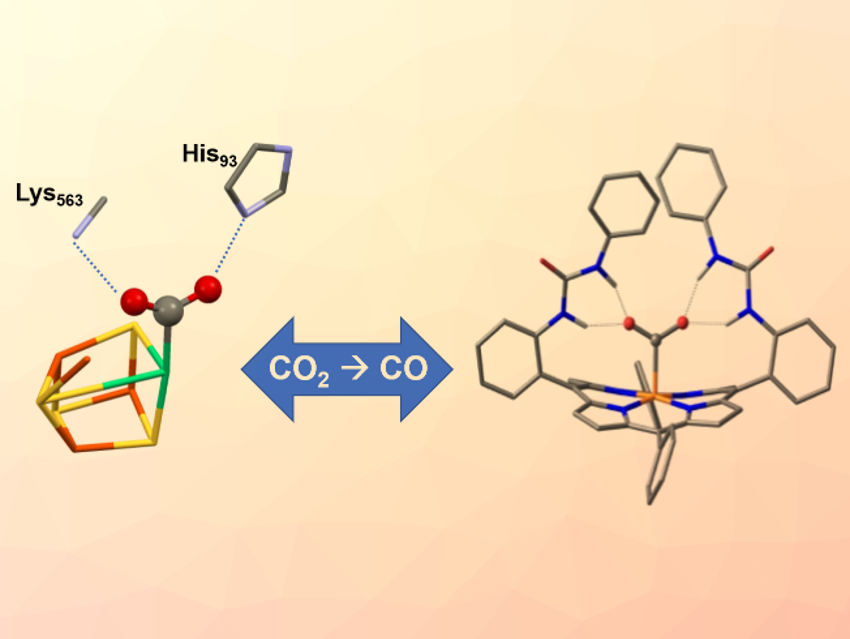
Nature has developed several enzymes to convert CO2 efficiently on a huge scale. In the enzyme carbon monoxide dehydrogenase (CODH), for example, a nickel-iron-based catalytic active site (pictured left) reversibly reduces CO2 to carbon monoxide (CO). The enzyme features two amino acids that hold the CO2 molecule in place through hydrogen bonds while electrons and protons are being added.
Ally Aukauloo, CEA Saclay, Gif-sur-Yvette, France, and Université Paris Sud, Orsay, France, Zakaria Halime, Université Paris Sud, and colleagues have modified an iron porphyrin catalyst (pictured right) with urea groups to lock a metal-bound CO2 in place by multiple hydrogen bonds. The resulting structure resembles the hydrogen‐bond stabilization scheme of the CO2 adduct in CODH.
Using this approach, the capture of CO2 is enhanced and less energy is needed to convert it to CO. The catalyst selectively generates CO with high Faradaic efficiency. These results might be beneficial for the development of cost-effective molecular catalysts for CO2 reduction.
- Second-Sphere Biomimetic Multipoint Hydrogen-Bonding Patterns to Boost CO2 Reduction of Iron Porphyrins,
Philipp Gotico, Bernard Boitrel, Régis Guillot, Marie Sircoglou, Annamaria Quaranta, Zakaria Halime, Winfried Leibl, Ally Aukauloo,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201814339