Chemically driven micro- and nanomotors have possible applications in, e.g., targeted drug delivery, environmental monitoring and remediation, or biosensing. However, the use of an external magnetic field to control their motion can be difficult in some of these applications. Building micro- or nanomotors that can sense changes in their local environment could be a better strategy to steer their movement.
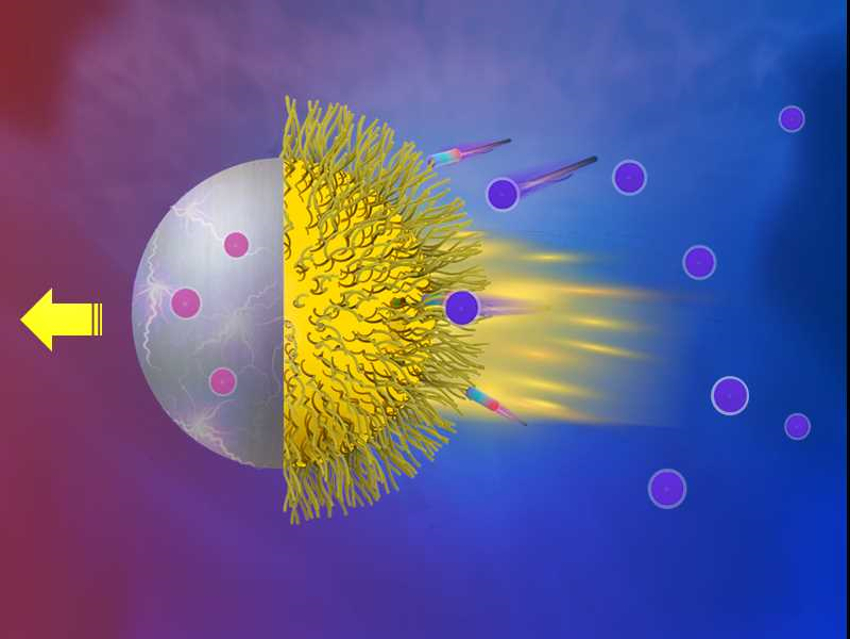
Yingjie Wu, Qiang He, Harbin Institute of Technology, China, and colleagues have developed a micromotor which consists of gold–platinum bimetallic Janus particles (i.e., particles with two different opposing surfaces). These particles were functionalized with thermoresponsive poly-N-isopropylacrylamide (PNIPAM) polymer brushes on the gold-covered half.
The micromotors are driven by a local electrostatic field. The field is caused by chemical reactions: the decomposition of H2O2 at the Pt surface generates protons, electrons, and O2, and at the Au surface, H2O2, protons, and electrons are consumed to produce H2O.
The PNIPAM@Au–Pt micromotor moved along the Au–Pt direction (pictured) with a speed of 8.5 μm/s in 1.5 % H2O2 at 25 °C. The micromotor changed the direction of motion (i.e., along the Pt–Au direction) and the speed decreased to 2.3 μm/s at 35 °C. This change is caused by a phase transition of the PNIPAM brush at 32 °C, which causes the polymer chains to collapse and hinders the reaction at the Au surface. According to the researchers, functionalization with thermoresponsive polymer brushes provides a new strategy for engineering the behavior of micro-/nanomotors.
- Thermoresponsive Polymer Brush Modulation on the Direction of Motion of Phoretically Driven Janus Micromotors,
Yuxing Ji, Xiankun Lin, Hongyue Zhang, Yingjie Wu, Junbai Li, Qiang He,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201812860