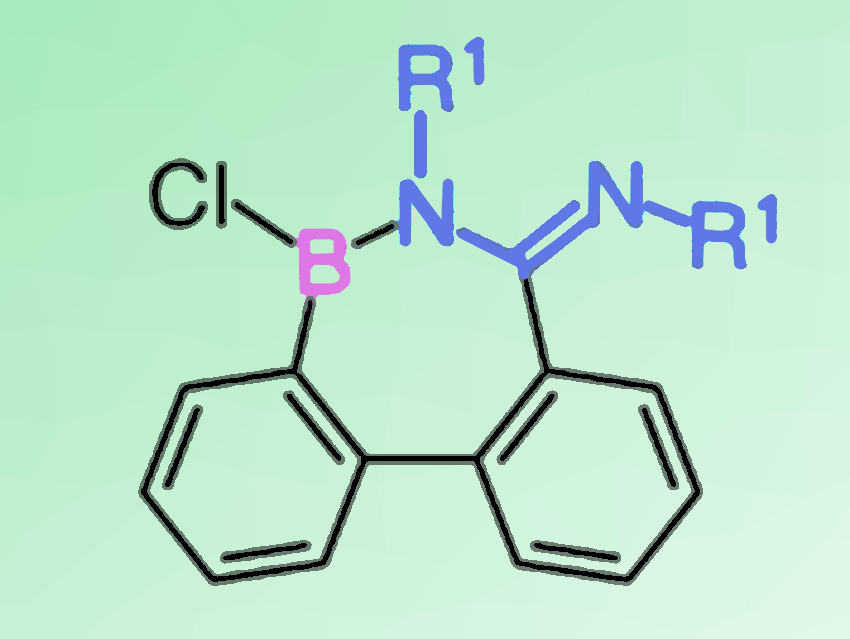
1,2-Carboboration reactions are characterized by the insertion of an unsaturated bond into a boron–carbon bond. When alkyne derivatives are used as substrates, this reaction is a useful tool for the regio- and stereoselective synthesis of substituted alkene derivatives.
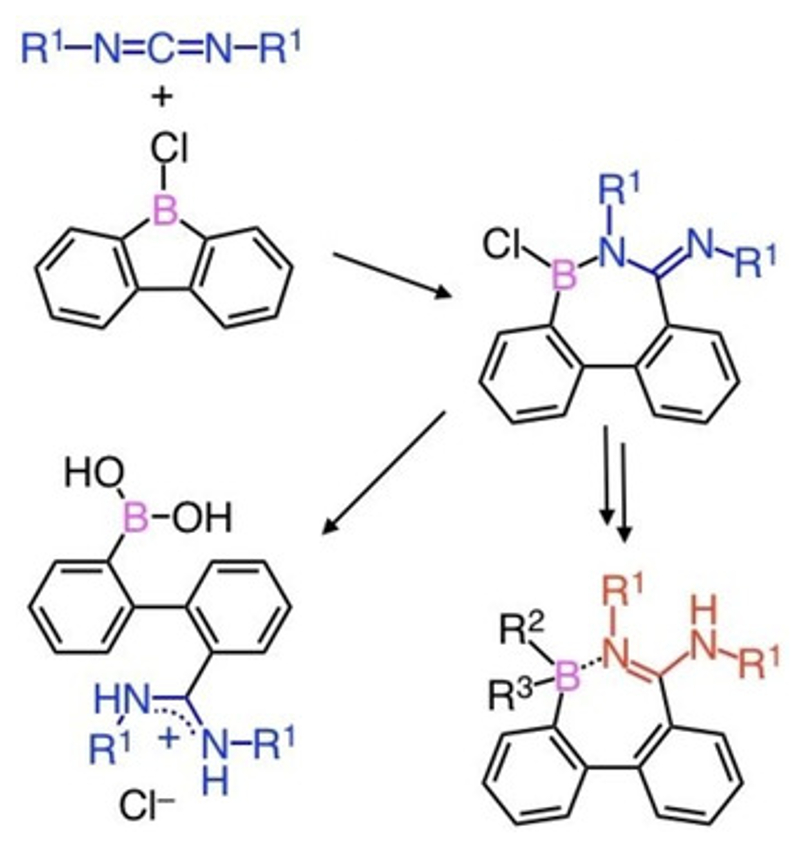
Junki Kashida, Yoshiaki Shoji, and Takanori Fukushima, Tokyo Institute of Technology, Yokohama, Japan, have found that a quantitative 1,2-carboboration reaction can take place between a C=N double bond of carbodiimide derivatives and 9-borafluorenes. The reaction gives a seven-membered ring compound (example pictured above) in which a tricoordinate borane and an amidine moiety are conjugated through a B–N bond. The BNC5 ring compound shows high stability toward air and water when its boron atom is protected with a bulky mesityl group.
Nucleophilic addition at the boron center of the BNC5 ring gives a tetracoordinate borate, while preserving the seven-membered ring structure (pictured below, bottom right). The BNC5 ring compound can also undergo a B–N bond cleavage upon hydrolysis, affording a biphenyl derivative with boronic acid and amidinate chloride functionalities (pictured below, bottom left). In the crystalline state, this bifunctional biphenyl forms an interesting one-dimensional chain-like structure via intermolecular and intramolecular hydrogen bonding. This unique molecule featuring both a Lewis acid (borane) and a conjugated base (amidine) may provide a new supramolecular building block with multiple molecular recognition sites.
- Synthesis and Reactivity of Cyclic Borane-Amidine Conjugated Molecules Formed by Direct 1,2-Carboboration of Carbodiimides with 9-Borafluorenes,
Junki Kashida, Yoshiaki Shoji, Takanori Fukushima,
Chem. Asian J. 2019.
https://doi.org/10.1002/asia.201900047