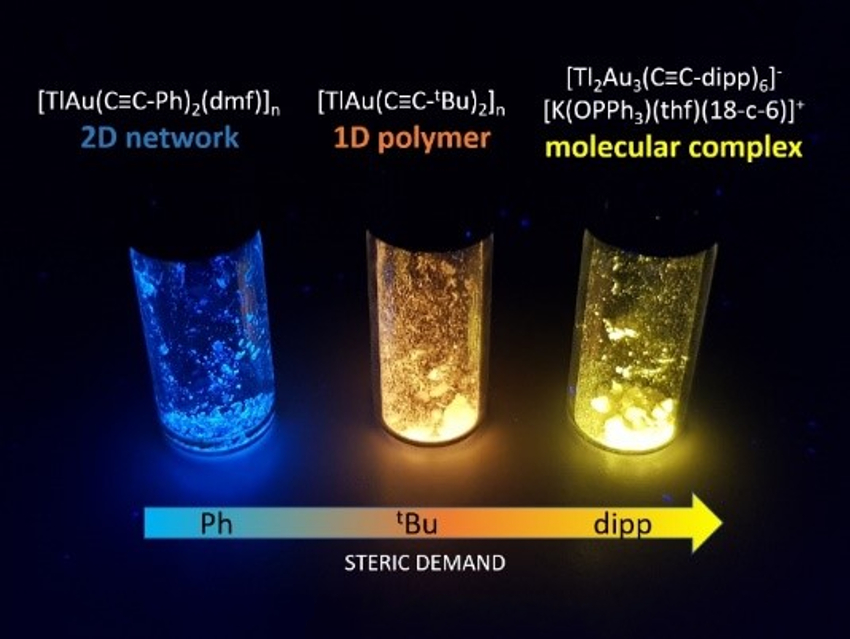
Supramolecular scaffolds with precious-metal backbones can have unusual structural features and luminescent behavior. Most of them are one-dimensional polymers, while related two- and three-dimensional networks are rare.
Peter W. Roesky, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have shown that the structure of such networks in can be controlled to a certain extent by the steric demand of the precursor. The team used synthesized AuI–TlI metallopolymers. They used bis(acetylido) aurates(I) of the type [K(thf)2(18‐crown‐6)][Au(C≡C−R)2] (thf = tetrahydrofuran) with varying steric demand in the R group as gold components. These compounds were reacted with thallium salts, i.e., thallium trifluoromethylsulfonate (TlOTf) or [Tl(OPPh3)3]OTf.
The researchers obtained 1D chains of interconnected dimers, tetramers, or dodecamers of AuI–TlI units, as well as a unique 2D network consisting of alternating (AuTl)6 and (AuTl)4 cycles. The formation of the different structures is primarily controlled by the steric demand of the acetylide substituent groups. Use of the bulkiest derivative, in which R = 2,6‐diisopropylphenyl (dipp), resulted in the formation of a zero-dimensional molecular cluster. Most compounds show bright visible photoluminescence at ambient temperature (pictured).
- Size Matters: From Two-Dimensional AuI -TlI Metallopolymers to Molecular Complexes by Simple Variation of the Steric Demand,
Tim P. Seifert, Nicolai D. Knoefel, Thomas J. Feuerstein, Kevin Reiter, Sergei Lebedkin, Michael T. Gamer, Andreas C. Boukis, Florian Weigend, Manfred M. Kappes, Peter W. Roesky,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201805984