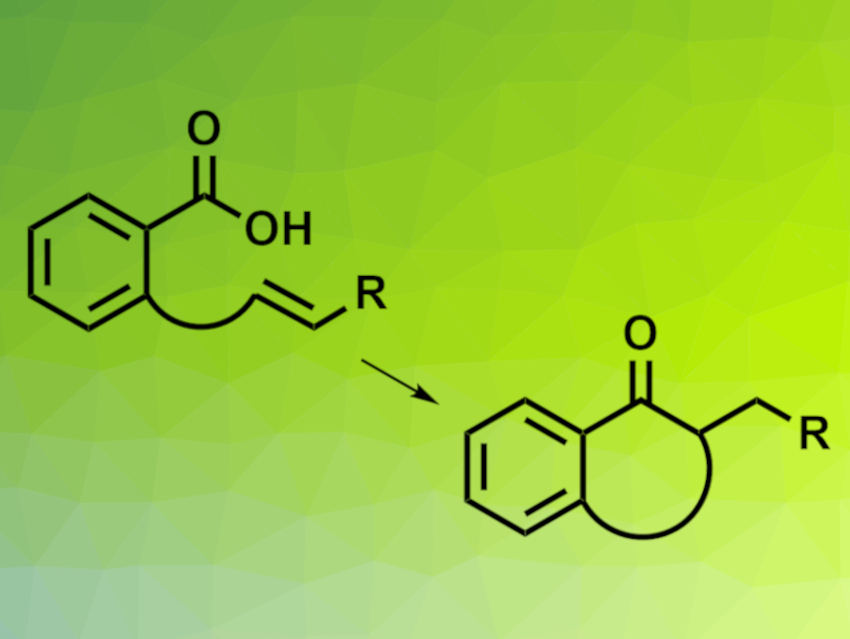
Carboxylic acids are abundant and inexpensive feedstock chemicals that can provide great structural diversity. Photoredox chemistry enables carboxylic acids to be used in acylation reactions. However, these reactions generally require stoichiometric activating reagents due to the inactivity of carboxylic acids.
Yoshiji Takemoto, Kyoto University, Japan, and colleagues have developed an intramolecular hydroacylation of olefins using carboxylic acids without a need for activating reagents (pictured below). The team developed a co-catalytic system consisting of boronic acid and an iridium photocatalyst. This allows carboxylic acids to be used directly as acyl radical precursors, leading to the conversion of carboxylic acid-containing substrates to the corresponding cyclic ketones. fac-Ir(dFppy)3 (dFppy = 2-(2′,4′-difluorophenyl)pyridine) was used as the photocatalyst and F3C–C6H4–B(OH)2 as the boronic acid. The reaction was performed in the presence of Hantzsch ester (HEH), γ-terpinene, and 5 Å molecular sieves in toluene under blue LED irradiation.
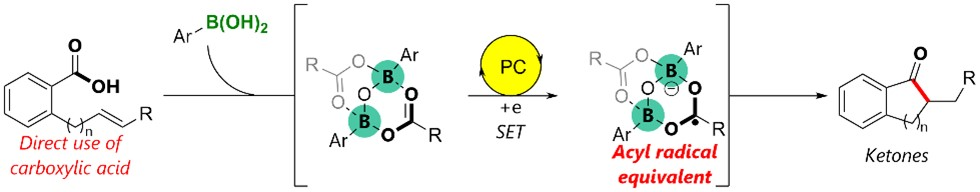
The team proposes a mechanism that involves a single-electron transfer to a carboxylic acid–boronic acid complex (pictured) and radical formation on the carbonyl carbon. This strategy could provide a useful tool for generating acyl radical equivalents.
- Boronic Acid‐Mediated Photocatalysis Enables the Intramolecular Hydroacylation of Olefins Using Carboxylic Acids,
Taichi Yumura, Takeshi Nanjo, Yoshiji Takemoto,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200082