Peptides play an important role in modulating biological processes, such as signaling and cell death. They could, thus, be useful in the pharmaceutical industry. There are a few dozen peptide therapeutics already on the market and over 150 in active clinical development. However, it is challenging to discover new peptide inhibitors for therapeutically relevant proteins.
Rainer Riedl, Zurich University of Applied Sciences, Wädenswil, Switzerland, and colleagues have discovered a peptide ligand which can inhibit the activity of a therapeutic target, matrix metalloproteinase 13 (MMP-13). MMPs are a family of zinc-containing enzymes that contribute to the degradation of extracellular matrix proteins. They are involved in diseases such as cancer.
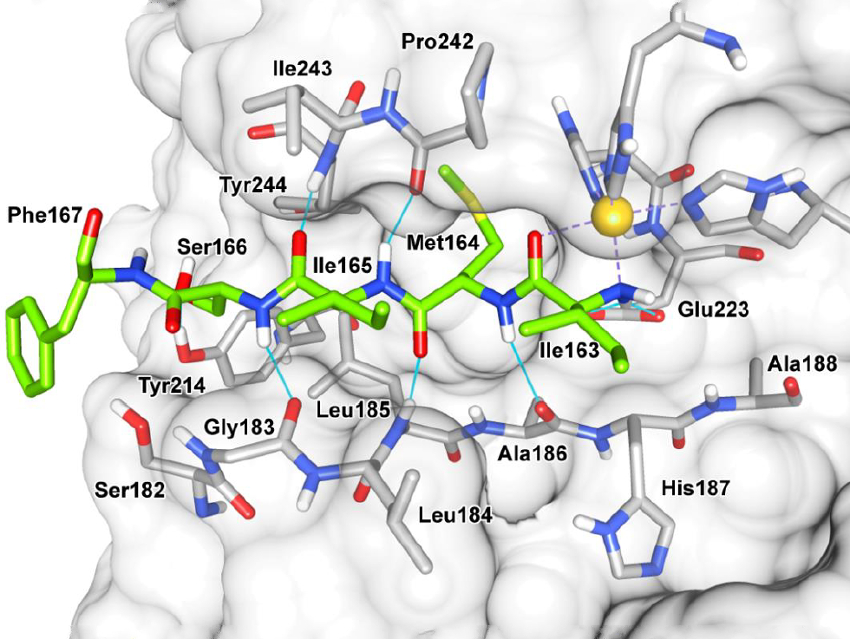
The team performed co-crystallization experiments with MMP-13 and small molecule inhibitors of the enzyme. Surprisingly, the team identified a pentapeptide ligand instead of the investigated inhibitors in the catalytic groove of MMP-13 (crystal structure pictured). This bound pentapeptide is a fragment of MMP‐13, created by self‐degradation of the enzyme.
Based on this discovery, the team developed optimized inhibitors with similar structures (peptidomimetics). They obtained a potent inhibitor which is membrane-permeable and shows a promising selectivity profile toward anticancer drug targets. The study demonstrates that crystallization studies can be useful for the discovery of protease ligands suitable for drug optimization.
- Drug Design Inspired by Nature: Crystallographic Detection of an Auto-Tailored Protease Inhibitor Template,
Flavio M. Gall, Deborah Hohl, David Frasson, Tobias Wermelinger, Peer R. E. Mittl, Martin Sievers, Rainer Riedl,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201812348