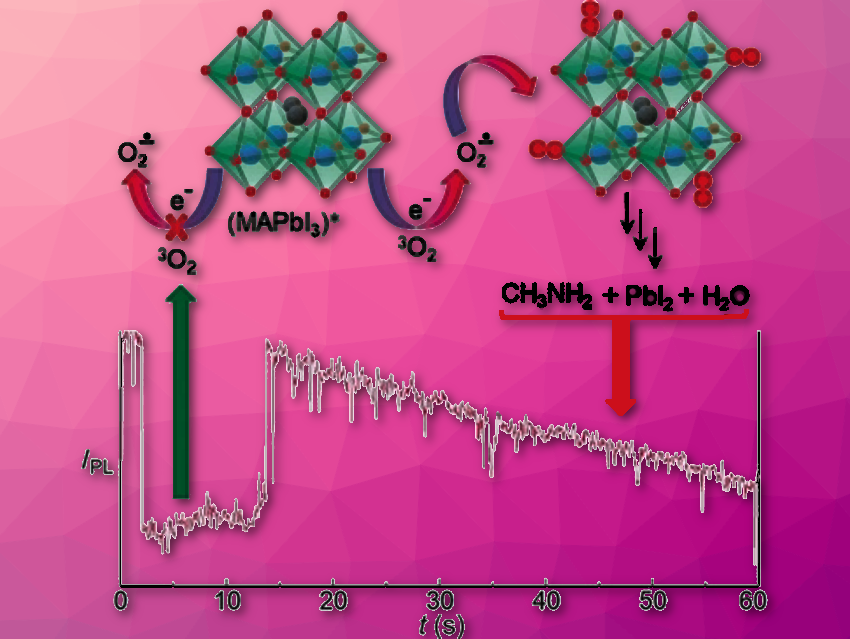
Lead halide perovskites are promising semiconducting materials for the manufacture of solar cells and LEDs. However, the structure and properties of these materials deteriorate when they are exposed to oxygen or moisture.
Vasudevanpillai Biju and colleagues, Hokkaido University, Sapporo, Japan, have discovered the origin of oxygen-sensitive degradation of methylammonium lead iodide (MAPbI3) perovskite nanocrystals. They analyzed the emission intensities of MAPbI3 single nanocrystals using single-molecule fluorescence microscopy and time-resolved fluorescence spectroscopy. The nanocrystals show stable emission under argon or embedded in a polymer. In the presence of oxygen, superoxide (O2–) forms, reacts with MAPbI3 (pictured right), and the emission intensity decreases.
Superoxide is only generated in the excited neutral state of MAPbI3. In the ionized state, MAPbI3 perovskites do not generate superoxide and do not degrade oxidatively. The researchers believe durability in perovskite-based devices could be controlled by using photovoltaic and electro-optical processes that are faster than electron transfer to oxygen.
- Blinking Beats Bleaching: The Control of Superoxide Generation by Photo-ionized Perovskite Nanocrystals,
Lata Chouhan, Sushant Ghimire, Vasudevanpillai Biju,
Angew. Chem. Int. Ed. 2019, 58, 4875–4879.
https://doi.org/10.1002/anie.201900061