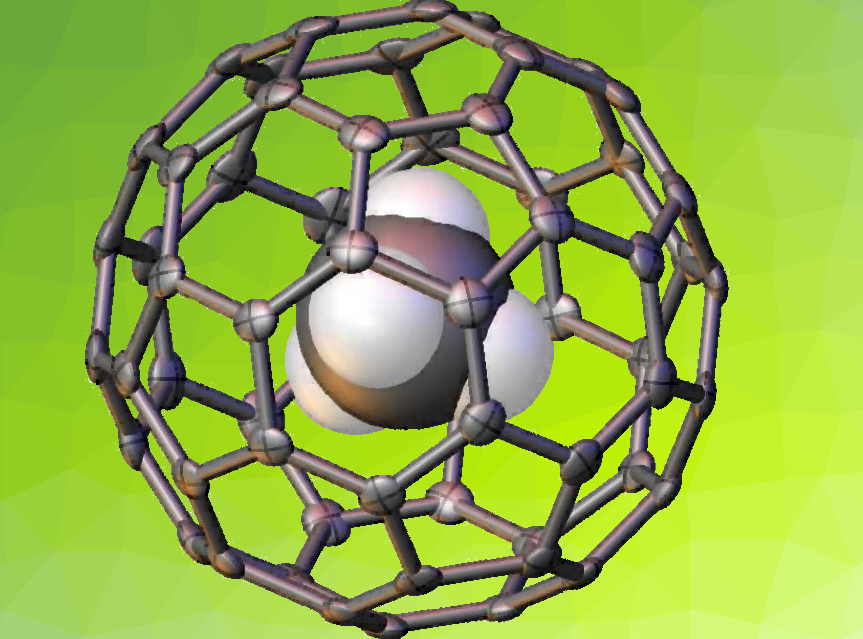
Buckminsterfullerene is a spherical cage of sixty carbon atoms that can be used to trap individual molecules. So-called endofullerenes allow an encapsulated molecule to be studied in an isolated environment.
Richard John Whitby, University of Southampton, UK, and colleagues have synthesized the first endofullerene containing a single molecule of methane, CH4@C60. Methane fills the internal cavity of C60. The researchers synthesized open-cage C60 with an aperture large enough to allow entry of a single methane molecule. Under 1645 atm of methane gas pressure, 95 % of the encapsulated species was formed. A series of reaction steps were then used to close the hole in the carbon cage.
CH4@C60 was studied with nuclear magnetic resonance spectroscopy (NMR), mass spectrometry (MS), and X-ray crystallography. The 1H spin-lattice relaxation times for the isolated methane molecule are similar to those in the gas phase, which suggests that the molecule is rotating freely inside the cage. The researchers believe that the encapsulation method could be used to synthesize novel endofullerenes containing small molecules such as CO2 or NH3.
- First Synthesis and Characterization of CH4@C60,
Sally Bloodworth, Gabriela Sitinova, Shamim Alom, Sara Vidal, George R. Bacanu, Stuart J. Elliott, Mark E. Light, Julie M. Herniman, G. John Langley, Malcolm H. Levitt, Richard J. Whitby,
Angew. Chem. Int. Ed. 2019, 58, 5038–5043.
https://doi.org/10.1002/anie.201900983



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)