Difluoroacetates are versatile precursors for the synthesis of difluoromethyl compounds or for the introduction of 18F labels. Thus, they have applications in drug discovery and total synthesis.
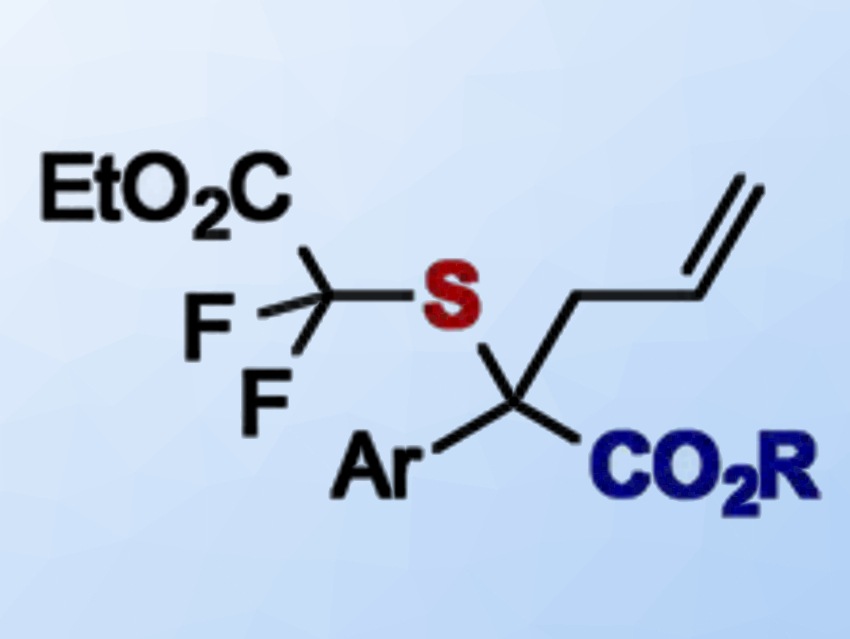
Sripati Jana and Rene M. Koenigs, RWTH Aachen University, Germany, have developed a protocol that allows the introduction of a difluoroacetate-thioether into diazoesters (product pictured). The team used a readily available RhII catalyst, Rh2(OAc)4, to promote a reaction between a diazoacetate and an allylic sulfide bearing a difluoroacetate substituent.
The diazoacetate acts as a carbene source. The carbene then undergoes an addition reaction with the sulfide compound and forms an ylide intermediate. This ylide undergoes a [2,3]‐sigmatropic rearrangement to give the product. This type of reaction is called a Doyle–Kirmse rearrangement.
The desired fluorinated homoallylic sulfides were obtained in good to excellent yields (up to 91 %). The reaction can also be performed under metal‐free conditions if visible light is used to generate the carbene intermediate.
- Doyle–Kirmse Rearrangement Reactions of Difluoroacetates,
Sripati Jana, Rene M. Koenigs,
Asian J. Org. Chem. 2019.
https://doi.org/10.1002/ajoc.201900099