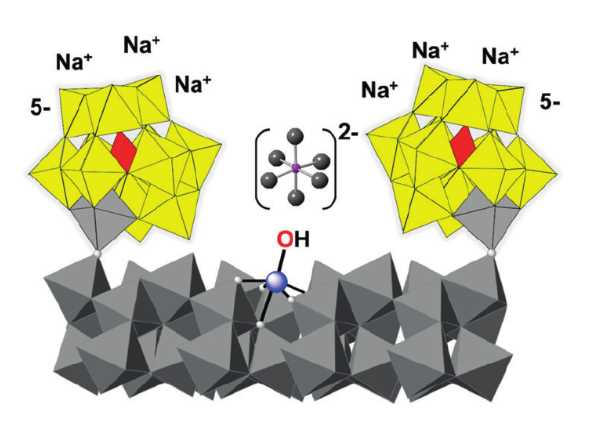
Metal-oxide nanocrystals, e.g., those composed of hematite (α‐Fe2O3), can be highly active water-oxidation catalysts. However, such nanoparticles often spontaneously aggregate in water. This usually prevents detailed mechanistic investigations.
Ira A. Weinstock, Ben-Gurion University of the Negev, Beer Sheva, Israel, and colleagues have used oxidatively inert polyoxometalate (POM) anions as ligands for a hematite core containing 275 iron atoms (surface pictured): The team reacted a POM of the type [α‐PW11O39FeIII]4−‐O− with amorphous γ-FeO(OH) at 220 °C.
The resulting clusters are only 3 nm in diameter but large enough to retain the photochemical properties of bulk hematite. One cluster is complexed by 15 POM molecules. The complex can catalyze visible‐light‐driven water oxidation for an entire week with no decrease in activity.
The solubility and stability of the hematite complex in water allowed the researchers to investigate the visible-light-driven water oxidation using solution-state methods. They found that water oxidation over the clusters occurs by a chain mechanism that is rate-limited by the capture of photoexcited electrons. In contrast, for hematite photoanodes the oxygen–oxygen bond-formation step is rate-limiting. The findings provide new insight into reactivity occurring at the surfaces of metal oxide photoanodes and multi-electron processes catalyzed by soluble metal oxide clusters.
- Visible-Light-Driven Water Oxidation with a Polyoxometalate-Complexed Hematite Core of 275 Iron Atoms,
Biswarup Chakraborty, Gal Gan-Or, Yan Duan, Manoj Raula, Ira A. Weinstock,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201900492