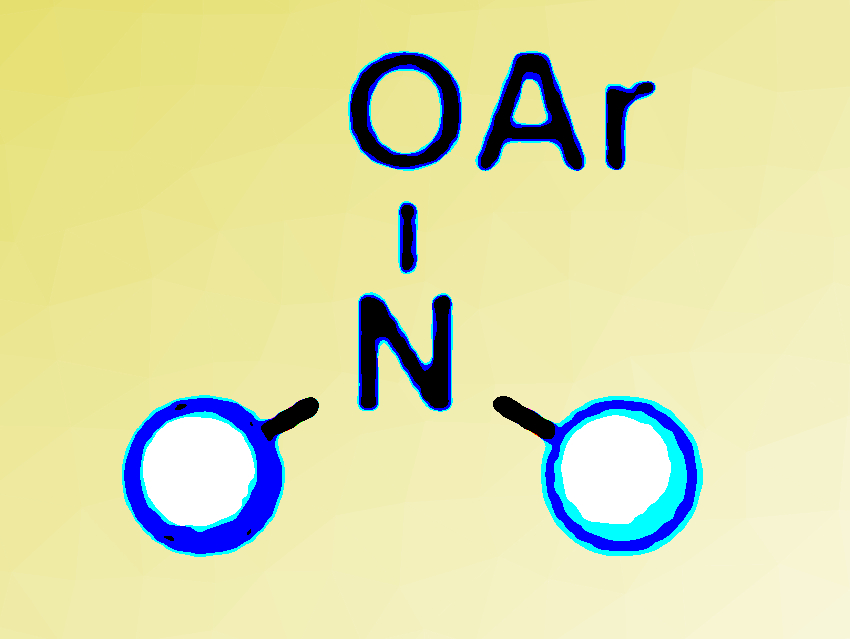
Nitrogen-containing compounds are the structural basis of many pharmaceuticals and agrochemicals. Consequently, efficient methods for the synthesis of such molecules are valuable tools in organic synthesis.
Daniela Leonori, University of Manchester, UK, and colleagues, have developed a method that produces synthetically useful nitrogen radicals. The researchers used aryl-NiI species to reduce electron-poor O-aryl hydroxylamine derivatives (pictured above) by a ground-state single-electron transfer mechanism to generate the radicals.
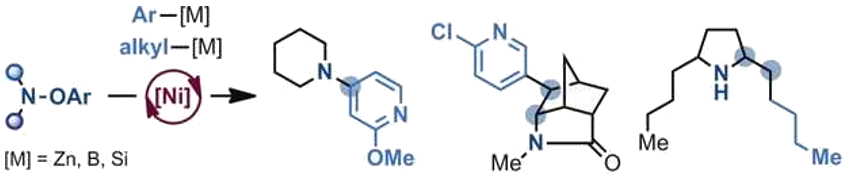
The N-radicals can react with a broad range of organometallic species, including boronic acids. The reactivity of the N-radicals is influenced by their structure: Electron-rich radicals form C–N bonds directly with aryl organometallic reagents to generate anilines. Electron-poor radicals undergo cascade processes that involve intramolecular cyclization and reaction with the organometallic coupling partner. (Examples of the products are pictured below).
Through a nickel-catalyzed cascade cyclization–alkylation of amidyl radicals, the team synthesized a series of alkaloids, including venom alkaloids found in ants and frogs. According to the researchers, a range of nitrogenated molecules could be accessed with the method.
- Reaction of Nitrogen-Radicals with Organometallics Under Ni-Catalysis: N-Arylations and Amino-Functionalization Cascades,
Lucrezia Angelini, Jacob Davies, Marco Simonetti, Laia Malet Sanz, Nadeem S. Sheikh, Daniele Leonori,
Angew. Chem. Int. Ed. 2019, 58, 5003–5007.
https://doi.org/10.1002/anie.201900510