The Roman god for whom the month of January is named, Janus, is a two-faced character—looking at once to the past and to the future. The god’s name was, thus, the obvious choice for “Janus” particles with two distinct opposite faces. It is usually not a useful term for molecular chemical entities, which are rarely spherical—with the notable exception of the C60 fullerene molecule, colloquially known as the buckyball.
Marius Kunkel and Sebastian Polarz, University of Konstanz, Germany, have developed an efficient one-pot synthesis for fullerenol derivatives which have Janus-like character (pictured). The team has also undertaken detailed characterization of these molecular entities.
Pole to Pole
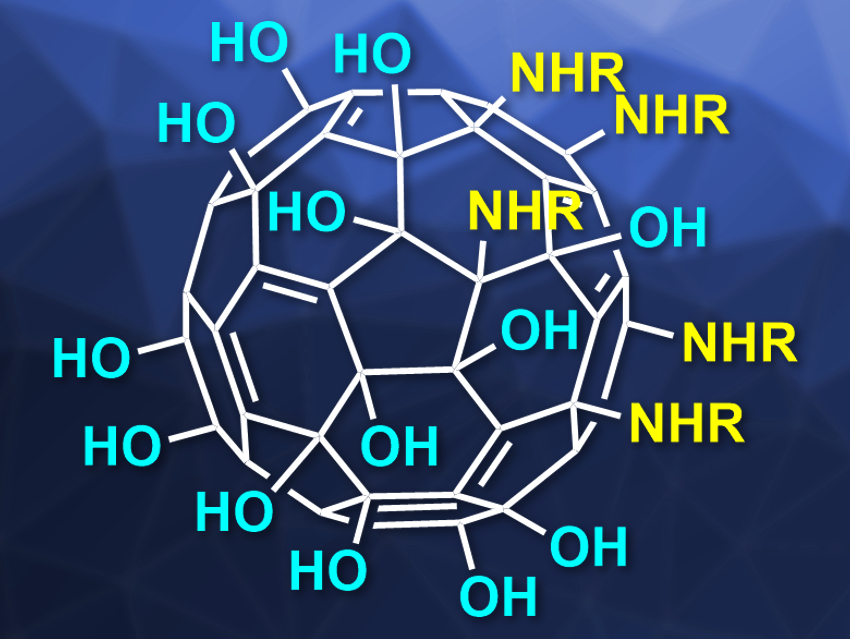
The team’s approach allows them to synthesize asymmetrically substituted fullerenol molecules with five substituents on one pole of the fullerene core. The remainder of the fullerene structure is covered with ether and hydroxy groups, thus, creating a two-faced buckyball. The team used a wide range of primary amines to generate Janus-type amphiphilic fullerenols with decent chemical yields. Ultimately, “these fullerenol amphiphiles can serve as suitable precursors for further reactions resulting in new applications for fullerenols,” the researchers state.
Fullerenes have a lot of potential in chemistry, biological, and materials sciences. Over the years, techniques have been developed to functionalize them, make them water-soluble, and generate structures with very different properties from the standalone buckyball, but retaining some of the rather useful characteristics of the truncated icosahedron that is the C60 molecule. Hydroxylated and polyhydroxylated compounds, termed the fullerenols, have become useful starting materials for functionalization. Adjusting the level of hydroxylation can be used to fine-tune solubility in polar and organic solvents, as well as in water. Moreover, the hydroxyl group is useful for further functionalization.
Convoluted Synthesis Leads the Way
In 2018, the team had synthesized a fullerenol derivative with up to 21 hydroxy groups on one hemisphere and 5 alkyl chains on the other [1]. However, the synthesis was very convoluted and gave them a rather low yield. Nevertheless, it was worth the effort, the team says, since they could improve the compound’s properties and pave the way to new applications for the system by using its amphiphilic behavior. Still, they were unable to attach more complex, and potentially useful, substituents instead of simple alkyl chains. The next step was to reduce the synthetic complexity and to take back control of both faces of their Janus buckyball.
Their new approach starts with a buckyball carrying six chlorine atoms. A common phase-transfer reaction let them combine water-soluble substituents with the precursor to the hexachlorofullerene. They could, thus, get high yields of a two-faced buckyball which has five defined substituents on one face in the initially chlorinated region of the surface, while the other “pole” of the sphere carries the requisite number of oxygen-containing moieties.
These modified fullerenols can act as surfactants. Moreover, the team adds, it is just one small step from this product to the addition of functional groups such as alkynes or bromides, which can be used as entry points to so-called “click chemistry” or Sonogashira coupling.
- Easy, efficient and versatile one-pot synthesis of Janus-type-substituted fullerenols,
Marius Kunkel, Sebastian Polarz,
Beilstein J. Org. Chem. 2019, 15, 901–905.
https://doi.org/10.3762/bjoc.15.87
Reference
- [1] Increasing the Resistance of Living Cells against Oxidative Stress by Nonnatural Surfactants as Membrane Guards,
Marius Kunkel, Stefan Schildknecht, Klaus Boldt, Lukas Zeyffert, David Schleheck, Marcel Leist, Sebastian Polarz,
ACS Appl. Mater. Interfaces 2018, 10, 23638–23646.
https://doi.org/10.1021/acsami.8b07032