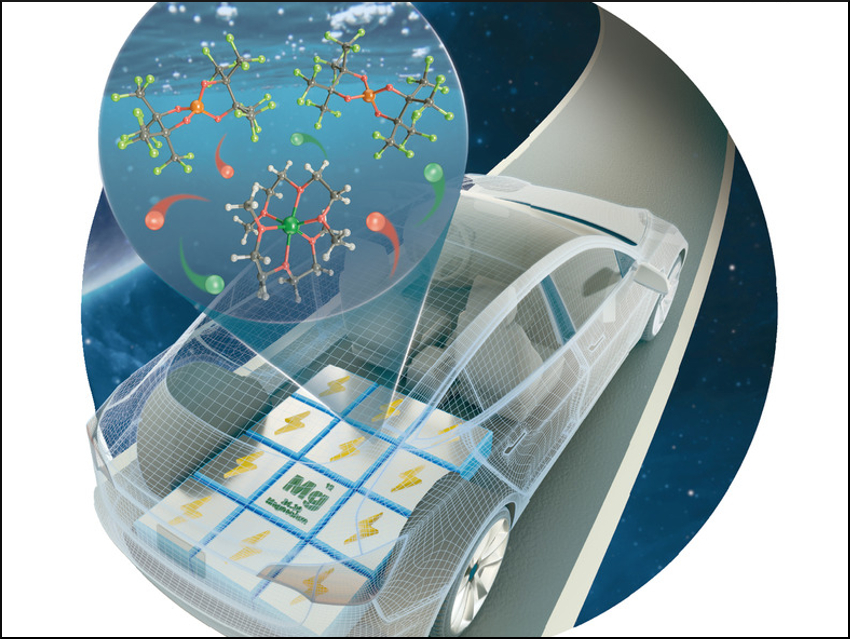
Magnesium is a promising anode material for rechargeable batteries. Its physicochemical properties rival that of lithium. Mg is environmentally benign, cheap, and it has a high capacity and reduction potential. Mg2+ electrolytes are an essential component of Mg batteries; however, such electrolytes typically contain chloride and are corrosive. Thus, these electrolytes are not chemically compatible with high-voltage cathode materials and commonly used current collectors such as steel or aluminium.
Tianbiao Leo Liu and colleagues, Utah State University, Logan, USA, have developed a stable, non-corrosive perfluorinated pinacolatoborate Mg electrolyte (Mg[B(O2C2(CF3)4)2]2 or Mg-FPB) for use in rechargeable Mg batteries. The team synthesized this chloride-free Mg salt in one step from commercially available Mg(BH4)2 and perfluorinated pinacol by heating a mixture of the reactants to 60 °C in dimethoxyethane (DME).
The non-coordinating pinacolatoborate anion interacts only weakly with the Mg2+ ion. This allows a fast electrochemical deposition and stripping of Mg2+, with an overpotential of 197 mV and a coulombic efficiency of 95 %. The oxidatively stabile Mg-FPB electrolyte can cycle for 500 hours and it withstands moisture. The non-corrosive electrolyte could be used in conjunction with common metallic current collectors such as stainless steel.
- A Stable, Non-Corrosive Perfluorinated Pinacolatoborate Mg Electrolyte for Rechargeable Mg Batteries,
Jian Luo, Yujing Bi, Liping Zhang, Xiaoyin Zhang, Tianbiao Leo Liu,
Angewandte Chemie International Edition 2019.
https://doi.org/10.1002/anie.201902009