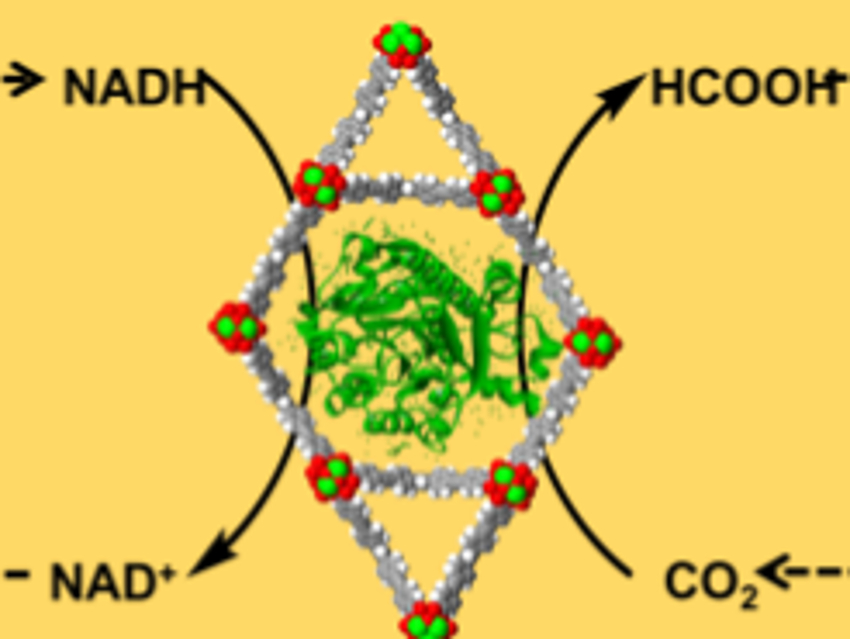
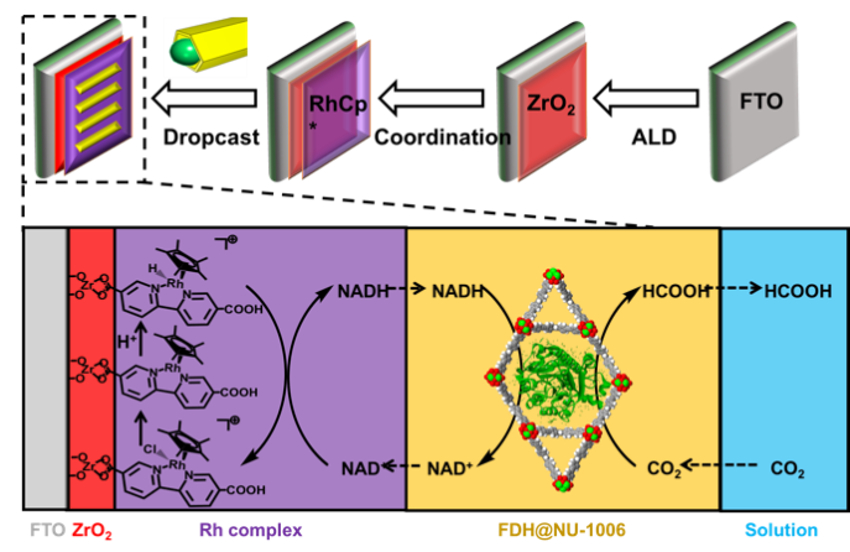
The use of enzymes can be an efficient route for carbon fixation. The enzyme formate dehydrogenase (FDH), for example, reduces carbon dioxide (CO2) to formic acid, coupled with the oxidation of the cofactor nicotinamide adenine dinucleotide (NADH) to NAD+. However, the enzymes are relatively unstable and hard to recycle, which limit the implementation of such processes. Metal-organic frameworks (MOFs), a class of porous crystalline materials, can be used as a stabilizing support for enzymes to prevent structural changes that affect their reactivity.
Omar K. Farha, Northwestern University, Evanston, IL, USA, and colleagues have synthesized a MOF called NU-1006 with large channels which can encapsulate FDH (pictured on yellow background). For the electrocatalytic conversion of NAD+ to NADH, a ZrO2-modified glass electrode (pictured in red) with a coordinated rhodium-based electron mediator (pictured in purple) was used. Drop-casting of the MOF-enzyme composite onto this rhodium-complex-modified electrode resulted in a complete bioelectrochemical reaction system (pictured below).
This system consistently provides FDH with NADH for the conversion of dissolved CO2 to formic acid. The MOF stabilizes FDH even under unfavorable conditions (for example, in an acidic environment), which can double the formic acid production compared to the free enzyme.
- Stabilization of Formate Dehydrogenase in a Metal-Organic Framework for Bioelectrocatalytic Reduction of CO2,
Yijing Chen, Peng Li, Hyunho Noh, Chung-Wei Kung, Cassandra T. Buru, Xingjie Wang, Xuan Zhang, Omar K. Farha,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201901981