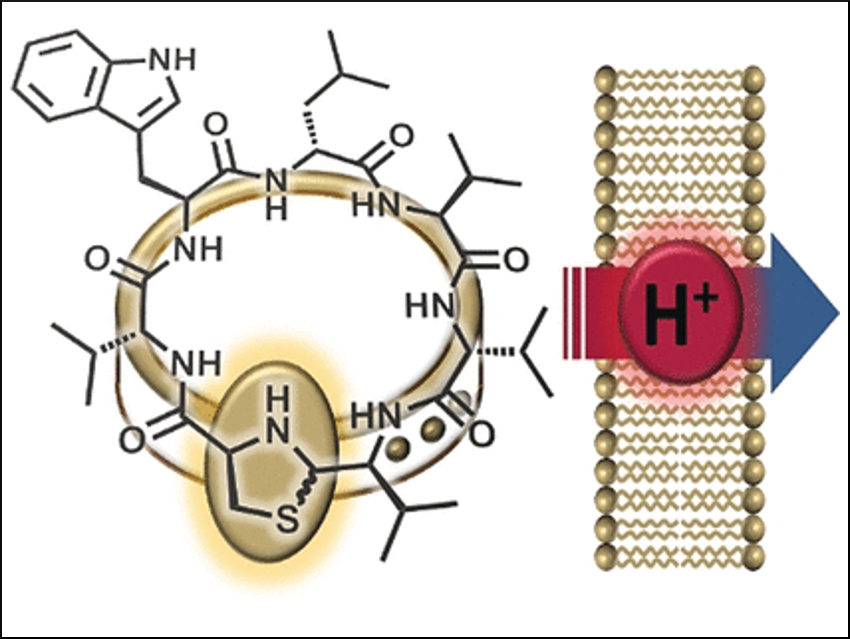
Peptide Antibiotic from the Human Nose
Antibiotic-resistant bacteria are an increasing health threat, making new antibiotics essential. German researchers have recently had a breakthrough: they discovered lugdunin (pictured) in the human nose—a new kind of cyclic peptide that comes from the bacterium Staphylococcus lugdunensis and has strong antimicrobial properties against Staphylococcus aureus, among others. The researchers have been able to clarify the mode of action by synthesizing variants. They found that proton transport across bacterial membranes is involved.
An interesting part of lugdunin’s structure is its thiazolidine group which forms a part of its peptide ring. This five-membered ring resembles a clasp that adorns the peptide ring. For this reason, the researchers named their new class of materials “fibupeptides”, from the Latin fibula, meaning clasp.
Lugdunin
Having previously succeeded in synthesizing lugdunin, Stephanie Grond, University of Tübingen, Germany, and colleagues have optimized the synthetic pathway to make many different derivatives of this natural substance. This allowed for a comprehensive study of the mechanism by which lugdunin works. The researchers made a series of derivatives in which they replaced each of the amino acids in the peptide ring with alanine, one in which they left off the “ornament clasp”, and a fibupeptide with a structure that is the mirror image of lugdunin. They then used these derivatives to carry out activity tests.
The team, consisting of chemists, biochemists, and microbiologists, discovered that the cyclic structure of the peptide, the thiazolidine “clasp”, and two amino acids (tryptophan and leucin), are critical to the antibiotic activity of the compound. In addition, the peptide ring must always be made of alternating D- and L-amino acids. However, there was no difference in the activity of the original molecule or its mirror image. “This indicates a lack of stereospecific receptor–ligand interaction,” states Nadine Schilling, a member of the team, “instead, it speaks for interaction with a small molecule or ion.”
Moving Protons across the Membrane
A further observation was that active lugdunin derivatives break down the electrical potential (the difference in voltage between the interior and exterior) of bacterial cell membranes, thereby killing the bacteria. Incorporating an additional tryptophan molecule intensified the interaction with the membrane and strengthened the antibacterial effect. Says Grond: “These results suggest ion transport across the bacterial membrane.”
To more closely examine this feature, the researchers produced synthetic vesicles with a pH gradient relative to the surrounding solution. Addition of active fibupeptides led to rapid pH equalization, without destruction of the membrane or formation of pores. “The mechanism clearly consists of translocation of protons across the membrane,” says Grond. “§We still need to determine whether lugdunin acts as a mobile transporter or a proton channel.”
- Synthetic Lugdunin Analogues Reveal Essential Structural Motifs for Antimicrobial Action and Proton Translocation Capability,
Nadine A. Schilling, Anne Berscheid, Johannes Schumacher, Julian S. Saur, Martin C. Konnerth, Sebastian N. Wirtz, José M. Beltrán-Beleña, Alexander Zipperer, Bernhard Krismer, Andreas Peschel, Hubert Kalbacher, Heike Brötz-Oesterhelt, Claudia Steinem, Stephanie Grond,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201901589