Boron compounds with thermally activated delayed fluorescence (TADF) could be useful for organic light-emitting diodes (OLEDs). However, the presence of a boron–carbon bond that is easy to break results in poor stability for these compounds. This usually makes them unsuitable for OLED applications.
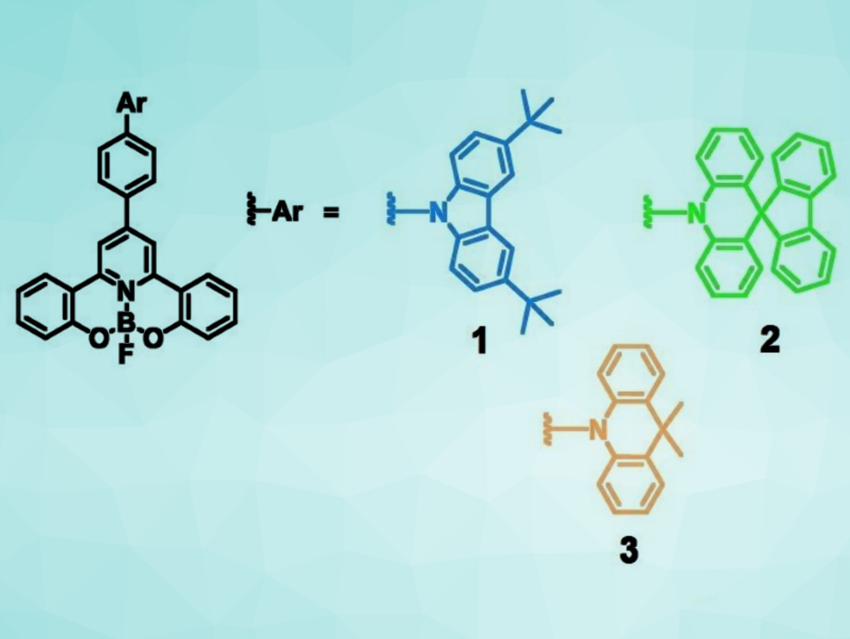
Mei‐Yee Chan, Vivian Wing‐Wah Yam, The University of Hong Kong, and colleagues have designed four-coordinate boron compounds (pictured) that could solve this problem. The team synthesized stable boron-containing TADF emitters by using tridentate chelating ligands that do not form boron–carbon bonds.
The emitters were prepared from 4‐Br‐Ph(dppy)Me2 (dppy = 2,2’‐(pyridine‐2,6‐diyl)diphenolate) using Buchwald‐Hartwig coupling reactions with either 3,6‐di‐tert‐butylcarbazole (DTC), 10H‐spiro[acridine‐9,9′‐fluorene] (FA), or 9,9‐dimethyl‐9,10‐dihydroacridine (DMAC). The compounds were then demethylated and reacted with BF3⋅OEt2. The three resulting compounds have emission colors from blue to yellow with luminescence quantum yields of up to 96 %.
High-performance OLEDs were built using these emitters. The devices have power efficiencies of 58.4 lm W–1 and external quantum efficiencies of 18 %. These OLEDs show long-term operational stability with half-lifetimes of more than 12,000 hours. These properties demonstrate the capability of four-coordinate boron compounds in optoelectronic devices.
- Four-Coordinate Boron Emitters with Tridentate Chelating Ligand for Efficient and Stable Thermally Activated Delayed Fluorescence Organic Light-Emitting Devices,
Panpan Li, Hing Chan, Shiu-Lun Lai, Maggie Ng, Mei-Yee Chan, Vivian Wing-Wah Yam,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201903332