The functionalization of unactivated C–H bonds is challenging but highly desired in organic synthesis. Unactivated C(sp2)–H and C(sp)–H bonds can be converted to various functional groups. However, this is more challenging for C(sp3)–H bonds due to their different reactivity. Most of the methods for C(sp3)–H functionalization require transition-metal catalysts, photocatalysts, or hypervalent iodine(III) reagents. However, these reagents are often expensive or require harsh reaction conditions.
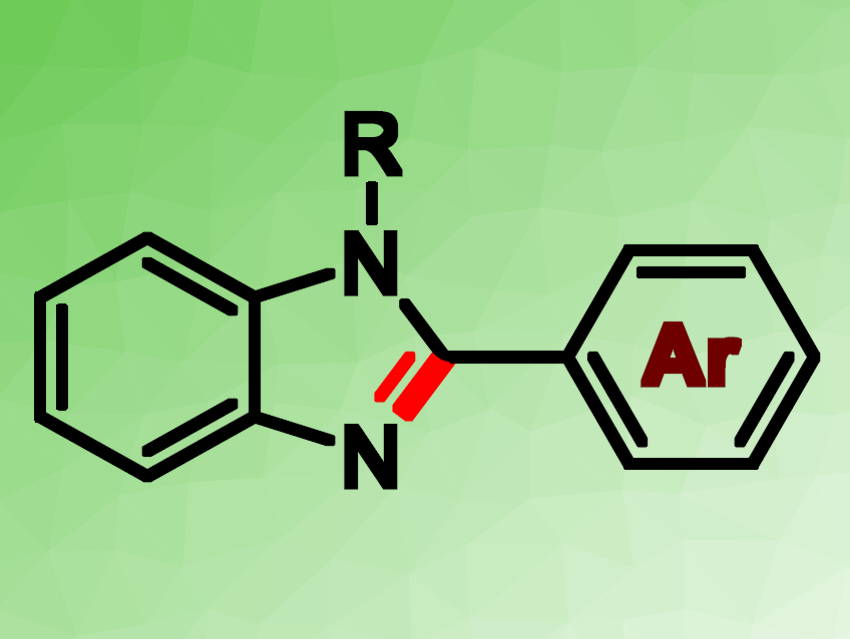
Prasenjit Mal and colleagues, National Institute of Science Education and Research (NISER), Bhubaneswar, India, have used the water-soluble, cheap iodine reagent tetrabutylammonium iodide (TBAI) as a catalyst for the functionalization of unactivated C(sp3)–H bonds in a benzylic group. The C–H bond was converted to an imine functionality, which allows the synthesis of benzimidazole rings (pictured). The desired transformation was achieved within 2 h using 20 mol % of TBAI, together with tert-butyl hydroperoxide (TBHP) as an oxidant, in dimethyl sulfoxide (DMSO),
Benzimidazole derivatives are useful in pharmaceutical chemistry. Thus, the researchers hope that their operationally simple, step-economic, and metal-free method for benzimidazole synthesis could have industrial applications.
- Intramolecular C(sp3)–H Imination using TBAI-TBHP,
Anima Bose, Sudip Sau, Prasenjit Mal,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900732



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)