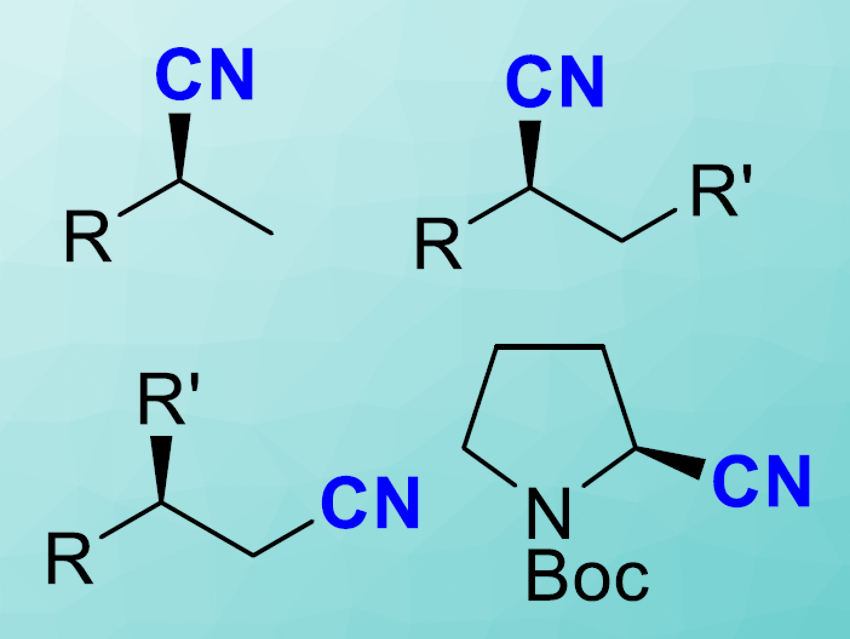
Nitriles are versatile building blocks in organic synthesis. In addition, some biologically active compounds bear a nitrile group. To make nitriles, synthetic chemists most commonly use a process called hydrocyanation, which essentially is the addition of hydrocyanic acid to alkenes. This process itself is rather straightforward, but the cyanide reagents are toxic. Xumu Zhang, Southern University of Science and Technology, Shenzhen, China, and colleagues have developed a convenient one-pot, two-step process to form nitriles from alkenes without involving cyanides. With their method, the team synthesized a variety of chiral nitriles, including several natural products.
Nylon and Chiral Nitriles
Hydrocyanation is an important synthetic reaction—for example, it is the first step in the megaton-scale Nylon-66 process. Here, cyanide is added to butadiene to form adiponitrile, which is then further processed to form the final product. Asymmetric hydrocyanation is also a great method to build chiral nitriles, which are highly useful intermediate compounds in organic synthesis. In this case, a cyanide reagent is added to a terminal alkene in the presence of a chiral transition metal catalyst to form the chiral nitrile.
The team looked for other, less toxic reaction pathways to nitriles. In principle, constructing the nitrile group without using cyanides is not a new thing. In 1993, a Spanish team already reported a synthetic route to nitriles starting from aldehydes [1]. In this procedure, a hydrazone was formed by adding a hydrazine to an aldehyde, and then the hydrazone was oxidatively cleaved to form the nitrile. This cleavage process was reported to be an aza-Cope elimination (not to be confused with the aza-Cope rearrangement, which is far more common—according to the number of Google results on both reactions, the elimination reaction does not seem to be used very often).
The problem with the hydrazone-elimination pathway is chirality. The hydrazone racemizes when it is subjected to follow-up reactions, which is also what Zhang and his colleagues observed: The chirality brought in by asymmetric hydroformylation of an alkene got lost when the aza-Cope elimination reagent, magnesium monoperoxyphthalate, was added. Finding appropriate conditions to avoid racemization, therefore, was the challenge the scientists faced.
Finding the Best Tandem Conditions
The key step in their synthesis was an asymmetric hydroformylation. Hydroformylation is the reaction of an alkene with carbon monoxide in the presence of a rhodium catalyst to form an aldehyde. If the rhodium catalyst is chiral, so is the aldehyde product, and the reaction is asymmetric. If a hydrazine is present in the reaction mixture as well, it reacts with the aldehyde, and the product is a chiral hydrazone. The scientists used styrene as a model alkene and 1-amino-piperidine as a model hydrazine (aromatic 1-methylphenylhydrazine did not give a product at all, so the researchers referred to aliphatic hydrazines).
The next step was the aza-Cope elimination, but this reaction led to the loss of chirality. The scientists identified unwanted dehydration and hydration side reactions as the source of racemization and tried to suppress them by making changes in the reaction conditions. Surprisingly, this task turned out to be not particularly difficult, and just using a more polar solvent (tetrahydrofuran instead of toluene) and benzoic acid as an additive directed the reaction to a satisfying 92 % enantiomeric excess of the final nitrile.
Scope and Progress
With their cyanide-free, convenient one-pot process in hand, the researchers studied the range of substrates and found that a quite impressive variety of different alkenes were suitable, most of them aromatic. Some aliphatic substrates such as limonene could also be transformed to the respective chiral nitriles. The team also demonstrated the synthesis of biologically active compounds, including ibuprofen, a common pain killer.
The researchers have not invented an entirely new pathway to make nitriles but they combined two processes, one well known, the other seemingly forgotten, to achieve hydrocyanation without handling toxic cyanide reagents. Synthetic chemists may now take more pleasure in synthesizing nitriles.
- Asymmetric Hydrocyanation of Alkenes without HCN,
Xiuxiu Li, Cai You, Jiaxin Yang, Shuailong Li, Dequan Zhang, Hui Lv and Xumu Zhanga,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201906111
Reference
- [1] Simple and efficient conversion of N,N-dimethylhydrazones and aldehydes to nitriles,
Rosario Fernández, Consolacíon Gasch, Jose-María Lassaletta, José-Manuel Llera, Juan Vázquez,
Tetrahedron Lett. 1993, 34, 141–144.
https://doi.org/10.1016/S0040-4039(00)60078-3