The activation and coupling of small molecules is a useful strategy for the synthesis of organic commodities (hydrocarbons, alcohols, ketones, or carbonic acids) and fine chemicals. One example for this is the bottom-up synthesis of heteroatom-containing organic molecules such as amines, amides, or cyanides from carbon oxides and ammonia as simple building blocks.
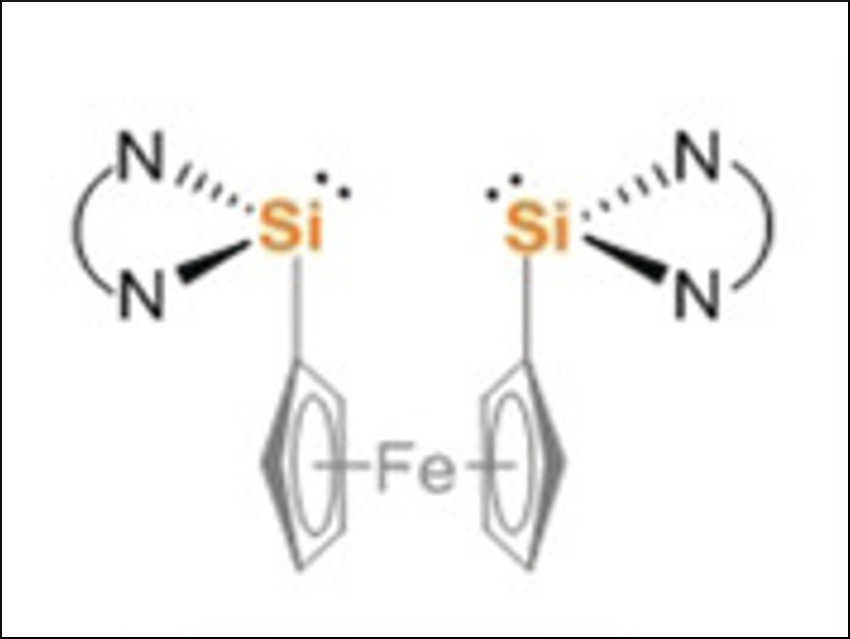
Matthias Driess and colleagues, Technische Universität Berlin, Germany, have developed a silicon-mediated direct coupling of carbon monoxide, ammonia, and primary amines to form acetamides. The transformation starts with the deoxygenative homocoupling of two CO molecules at two cooperative divalent silicon atoms in a bis(silylene) (pictured above). Ammonolysis of the resulting disilaketene (pictured below on the left) first causes the formation of an N-silylated carboxamide and then affords the desired acetamide and disilyldiamide.
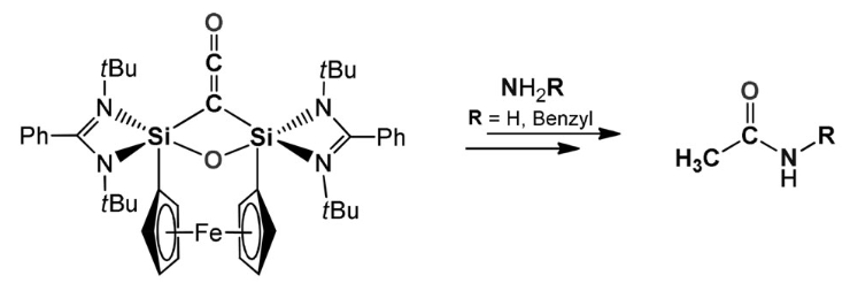
According to the researchers, this work could inspire the further development of coupling reactions of small molecules based on earth-crust-abundant, “green” elements such as silicon.
- Silicon-Mediated Coupling of Carbon Monoxide, Ammonia, and Primary Amines to Form Acetamides,
Marcel-Philip Luecke, Arseni Kostenko, Yuwen Wang, Shenglai Yao, Matthias Driess,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201904361