Organic reactions driven by visible light can be a powerful tool to build complex molecules. In this context, Ru(II) or Ir(III) photocatalysts are commonly used. Replacing these catalysts with environmentally friendly and much more abundant Cu-based complexes would be useful. However, most copper-based photocatalysts require ligands that are time-consuming and expensive to synthesize.
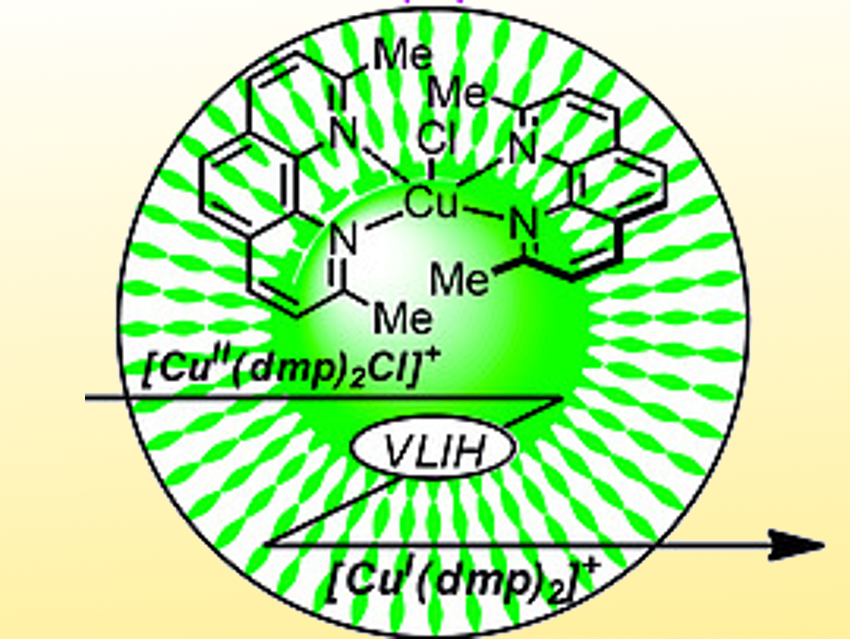
Sebastian Engl and Oliver Reiser, University of Regensburg, Germany, have found a Cu(II) complex that can act as a precursor for a Cu(I) photocatalyst. This approach is efficient but considerably more cost-effective compared to previously established Cu(I) complexes. The team found that the Cu(II)-complex [CuII(dmp)2Cl]Cl (pictured, dmp = 2,9-dimethyl-1,10-phenanthroline) can be used as an oxidation-stable and bench-stable precursor for visible-light-mediated Cu(I)-photocatalysis. The complex can be easily synthesized from CuCl2 and commercially available dmp.
The performance of the catalyst was demonstrated within a broad scope of atom transfer radical addition (ATRA) reactions, which can be used for the 1,2-difunctionalization of alkenes. The catalyst can also be used for decarboxylative couplings and Appel reactions, which convert alcohols to organic halides. In some cases, [CuII(dmp)2Cl]Cl even outperformed established Cu(I) catalyst systems.
- Making Copper Photocatalysis Even More Robust and Economic: Photoredox Catalysis with [CuII(dmp)2Cl]Cl,
Sebastian Engl, Oliver Reiser,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900839