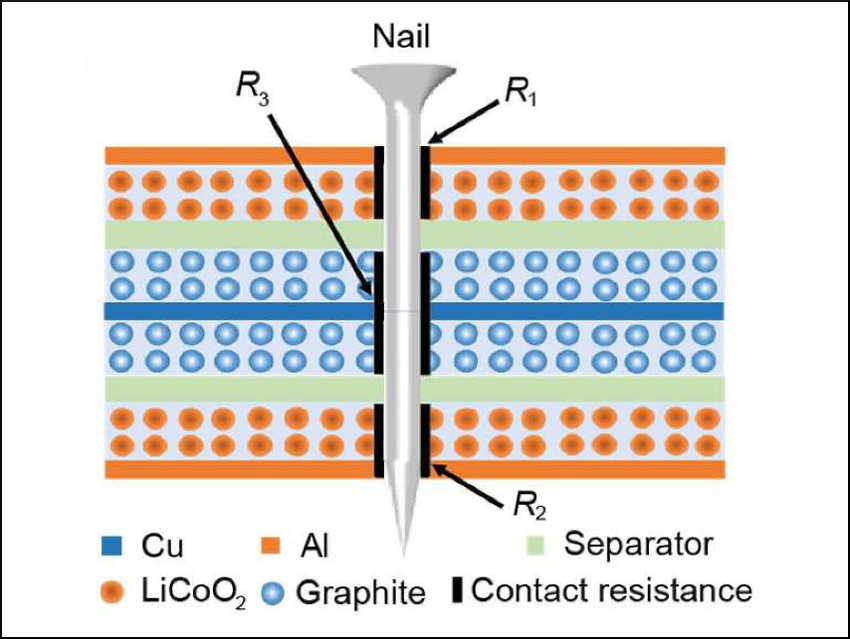
Lithium-ion batteries have a reputation for exploding when they are exposed to conditions like mechanical deformation, over-charging, or heat. This is due to the high flammability of the organic electrolyte. Nail penetration is often used as an extreme test for evaluating thermal runaway by inducing an internal short-circuit. The contact resistance between the nail and the electrodes leads to heat generation. So far, researchers have usually assumed a uniform resistance and have not measured it experimentally.
Yuan Yang, Columbia University, New York, USA, and colleagues have experimentally determined for the first time the heterogeneous short-circuit contact resistance between a nail and two different electrodes, a Cu/graphite electrode and an Al/LiCoO2 electrode. The resistances are 2.5±1.5 Ω and 20.3±12.4 Ω, respectively. The resistance varies throughout the battery. This heterogeneity can significantly increase the temperature rise compared to homogeneous contact resistance.
The resistance depends on the layer sequence: Heat generation in the top Al-based cathode layer (pictured in orange) is the highest among the battery layers. The researchers propose that having the Cu-based anode as the outermost layer in a battery stack can reduce heat generation during nail penetration. The results shed light onto a long-term myth in the battery community and provide direction for improving the safety of Li-ion batteries.
- New Insights into Nail Penetration of Li‐Ion Batteries: Effects of Heterogeneous Contact Resistance,
Meijie Chen, Qin Ye, Changmin Shi, Qian Cheng, Boyu Qie, Xiangbiao Liao, Haowei Zhai, Yurong He, Yuan Yang,
Batteries & Supercaps 2019.
https://doi.org/10.1002/batt.201900081