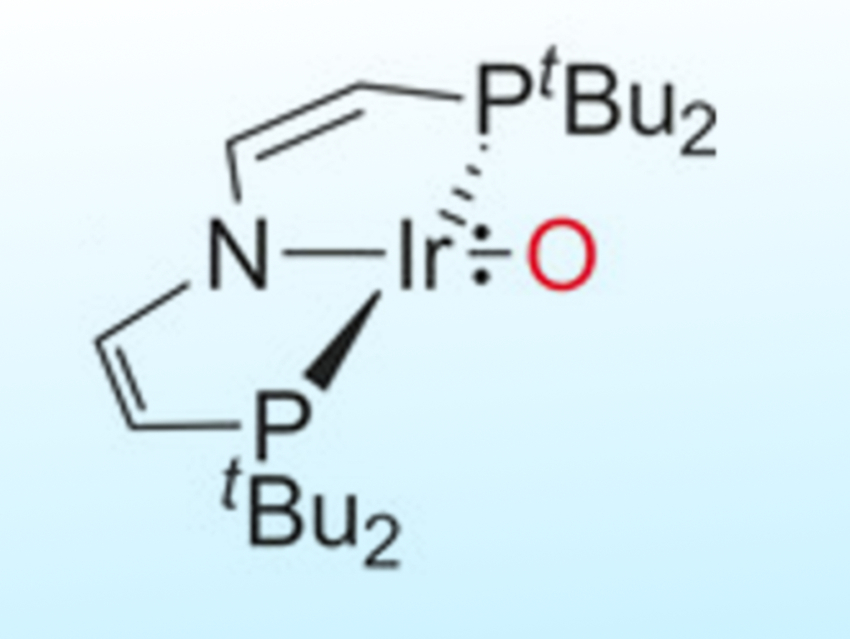
Transition-metal oxo complexes with multiply bonded ligands have low stability if their M=O antibonding orbitals are populated. Electron-rich compounds are considered key transient species in catalytic oxo transfer reactions. However, beyond group 8 metals, few well-characterized examples are known.
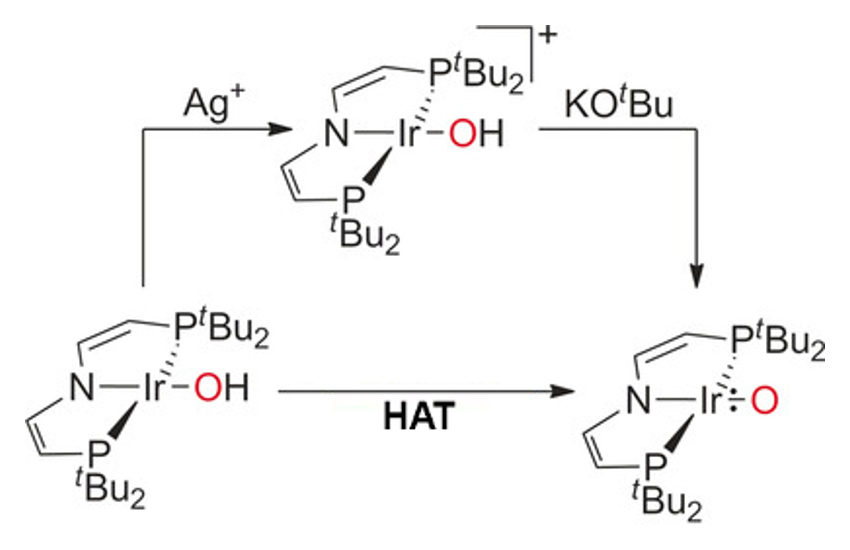
Sven Schneider, Universität Göttingen, Germany, Max C. Holthausen, Goethe-Universität Frankfurt, Germany, and colleagues have discovered an iridium complex (group 9) with a terminal oxo ligand stabilized by a bulky pincer framework (pictured above). The IrO{N(CHCHPtBu2} complex is synthesized by either deprotonation with potassium tert-butoxide or hydrogen atom transfer with 2,4,6-tris-tert-butylphenoxyl (pictured below). The complex possesses an unprecedented triplet ground state with two unpaired electrons delocalized equally within the Ir=O core.
This unusual bonding situation allows ambiphilic oxo transfer to nucleophiles, such as PMe3, and electrophiles, such as CO2. Calorimetric and computational examination of the bond dissociation energy of the IrO–H bond suggests that the oxo complex could activate strong C–H bonds, such as those found in hydrocarbons.
- A Terminal Iridium Oxo Complex with a Triplet Ground State,
Daniel Delony, Markus Kinauer, Martin Diefenbach, Serhiy Demeshko, Christian Würtele, Max C. Holthausen, Sven Schneider,
Angew. Chem. Int. Ed. 2019, 58, 10971–10974.
https://doi.org/10.1002/anie.201905325