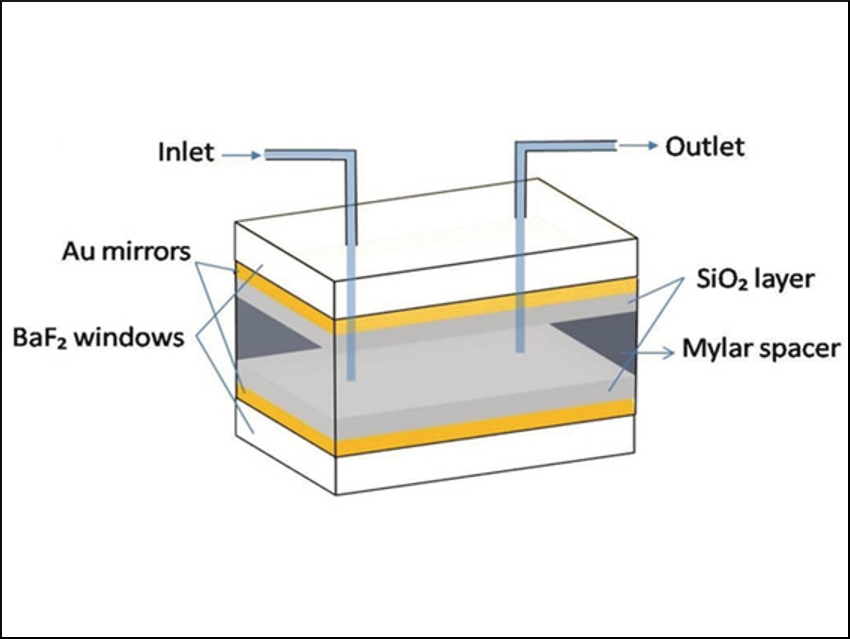
When talking of light-matter interactions, photochemistry immediately comes to mind. Surprisingly, light-matter interactions can modify chemical reactions even in the dark, using infrared (IR) waves. This unconventional approach involves strongly coupling the reactant’s vibrational bands to electromagnetic fluctuations in a microfluidic optical cavity consisting of two parallel mirrors (pictured). By tuning this so-called Fabry-Pérot (FP) cavity to the vibrational transition of interest, it can accelerate the chemical reaction. This concept is called cavity catalysis.
Jino George, Indian Institute of Science Education and Research, Mohali, Thomas W. Ebbesen, University of Strasbourg, France, and colleagues have shown that the solvolysis of the ester para-nitrophenylacetate (PNPA) with ethylacetate (EtOAc) as the solvent can be sped up by more than an order of magnitude by cavity catalysis. The team used FP cavities with Au mirrors sputtered onto BaF2. The spacing between the mirrors determines the frequency the cavity is tuned to.
The strong catalytic effect is based on a cooperative interaction: It is observed when the FP cavity is tuned to the C=O vibrational stretching mode of both the reactant and solvent molecules. The electron-density distribution in the reaction’s transition state is strongly modified, resulting in a more polar activated complex and faster reaction rates. Vibrational energy can be transferred from the solvent to the reactant, as long as their vibrational resonances overlap. According to the team, this cooperative effect could help further studies of cavity catalysis, because it removes the need for a high concentration of reactants to achieve strong coupling.
- Cavity Catalysis by Cooperative Vibrational Strong Coupling of Reactant and Solvent Molecules,
Jyoti Lather, Pooja Bhatt, Anoop Thomas, Thomas W. Ebbesen, Jino George,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201905407