Hydrogen generation through electrochemical water splitting is a promising way to reduce our dependence on fossil fuels. However, the oxygen evolution reaction (OER) is a bottleneck in this process. RuO2 and IrO2 are known state-of-the-art OER catalysts, but their high cost and poor durability limit their industrial application. Low-cost, earth-abundant electrocatalysts are needed for this important reaction.
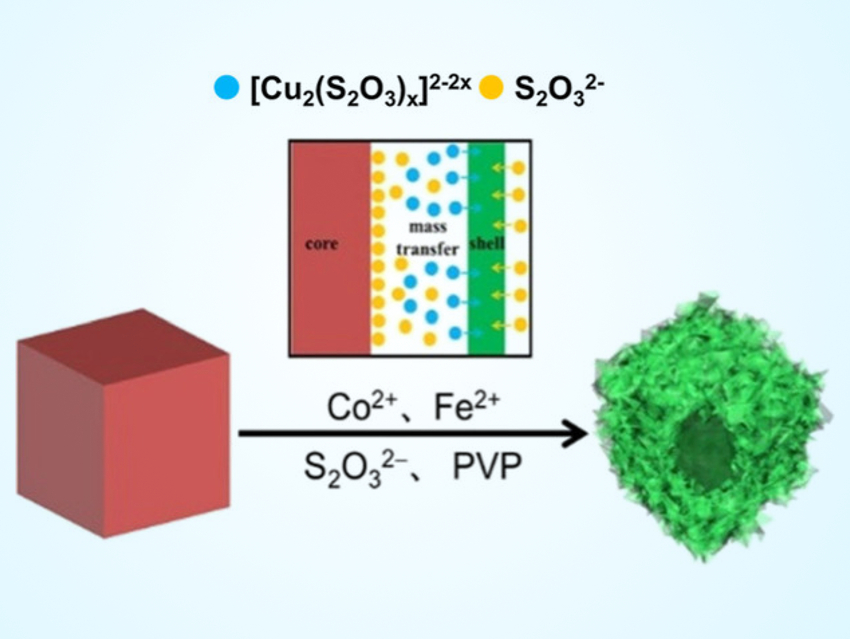
Rui Cao, Haoquan Zheng, Shaanxi Normal University, Xi’an, China, and colleagues have developed hierarchical hollow nanocubes based on ultrathin Co–Fe layered double hydroxide (CoFe–LDH) nanosheets. First, the researchers prepared Cu2O nanocubes (pictured in red) as self-sacrificing templates. Then, they used these templates to synthesize hollow nanocubes (pictured in green) consisting of CoFe–LDH nanosheets by a process called coordinating etching.
Solutions of Co and Fe species as well as Na2S2O3 and polyvinyl pyrrolidone (PVP) were added to the Cu2O nanocubes. At the interface between Cu2O and the solution, Cu2O is converted into [Cu2(S2O3)x]2−2x, which dissolves in water. S2O32− can produce OH−, which causes the precipitation of the desired CoFe−LDHs (process pictured above).
The scientists found that the hollow nanocubes can effectively catalyze water oxidation at a low overpotential of 270 mV and a current density of 10 mA cm–2. The catalysts also showed long-term stability in an aqueous KOH solution. The work offers a simple strategy for preparing dual-metal hollow nanocubes with abundant active sites and tuned properties for the OER.
- Ultra-thin Co–Fe Layered Double Hydroxide Hollow Nanocubes for Efficient Electrocatalytic Water Oxidation,
Haitao Yuan, Yanzhi Wang, Chenxi Yang, Zuozhong Liang, Mingxing Chen, Wei Zhang, Haoquan Zheng, Rui Cao,
ChemPhysChem 2019.
https://doi.org/10.1002/cphc.201900524