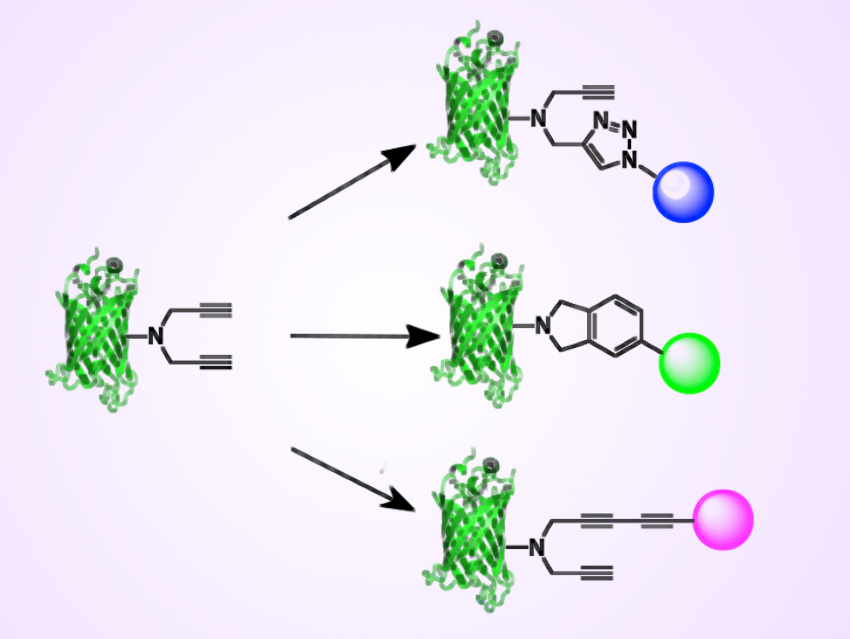
Bioconjugation reactions, i.e., linking a biological molecule to another chemical compound, are widely used in therapeutic, diagnostic, and materials applications. Thus, the development of new reactions for the preparation of bioconjugates that can be performed under biological conditions is useful. Often, new chemical “handles” must be introduced to biological systems to prevent undesirable side reactions during bioconjugation. One mechanism to introduce such handles into proteins is using unnatural amino acids (UAAs).
Douglas Dean Young, College of William & Mary, Williamsburg, VA, USA, and colleagues have synthesized and genetically encoded a UAA with a dipropargyl amine moiety (pictured left). The team encoded this UAA into green fluorescent protein (GFP). The amino acid was then used in a [2+2+2] cyclotrimerization reaction with an alkyne-containing partner (pictured center right).
This represents a new bioconjugation reaction. The UAA can also be used in other, previously developed bioconjugation reactions, including the 1,3-dipolar cycloaddition reaction with an azide (pictured top right), and the Glaser-Hay coupling with another alkyne (pictured bottom right). According to the researchers, this UAA could be used to make protein-drug conjugates for the treatment of various diseases or to immobilize proteins for materials applications.
- Genetic encoding of a bioconjugation handle for [2+2+2] cycloaddition reactions,
Christopher Travis, Gillian Gaunt, Elizabeth King, Douglas Dean Young,
ChemBioChem 2019.
https://doi.org/10.1002/cbic.201900391