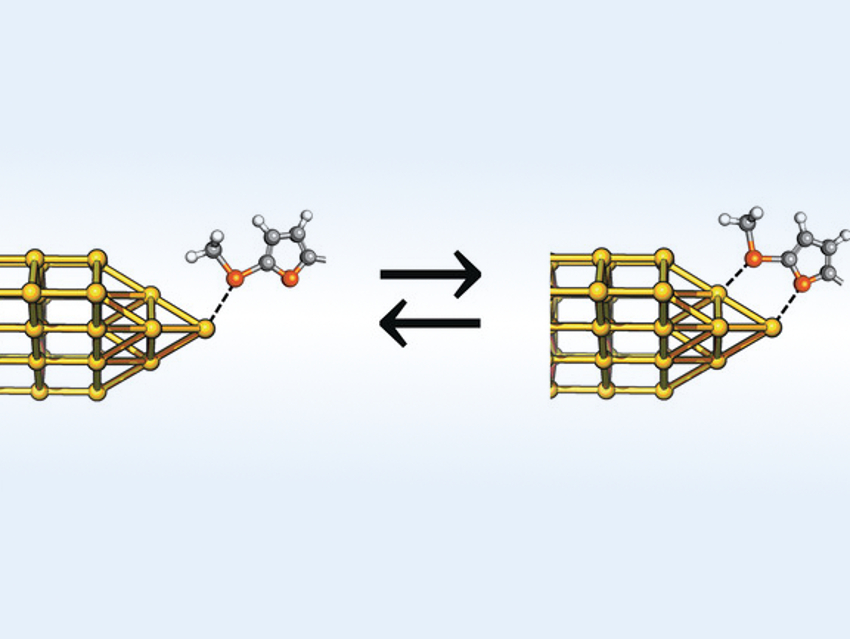
Molecules with a conductance that is “gated” by external stimuli (e.g., light or an external potential) can be useful in molecular electronics. Andrea Vezzoli, Simon J. Higgins, University of Liverpool, UK, Sara Sangtarash, Lancaster University and University of Warwick, UK, Colin J. Lambert. Lancaster University, and colleagues have developed another mode of conductance control. The team designed molecules whose conductance increases sharply upon a change in their binding mode to metal contacts (pictured). This uses a concept known from homogeneous catalysis: the hemilability of bidentate ligands with a strongly and a weakly binding group.
The team designed conjugated molecules (“molecular wires”) with (2-methylthio)thiophene units at the end as contacts. These molecular wires can interact with a metal electrode, e.g., a gold substrate. The junction between the thiophene groups and the electrode can be modified using a scanning tunneling microscopy (STM) technique. The team observed conductance changes of up to two orders of magnitude as the junctions were compressed and stretched by moving the microscopy tip.
The researchers explain this observation by additional weak contacts between the thiophene’s sulfur atoms and gold electrodes that occur only at short tip–substrate distances, i.e., there is a monodentate→bidentate transition when the distance is reduced. This additional interaction increases the conductance. Building such features into molecules offers a new method to control their electrical properties.
- Hemilabile ligands as mechanosensitive electrode contacts for molecular electronics,
Nicoló Ferri, Norah Algethami, Andrea Vezzoli, Sara Sangtarash, Maeve McLaughlin, Hatef Sadeghi, Colin J. Lambert, Richard J. Nichols, Simon J. Higgins,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201906400