The activation and transformations of C–S bonds in thioether compounds is useful, e.g., in the petroleum industry and in organic chemistry. Such reactions usually rely on the insertion of a transition-metal catalyst into the C−S bond, leading to C−S bond cleavage.
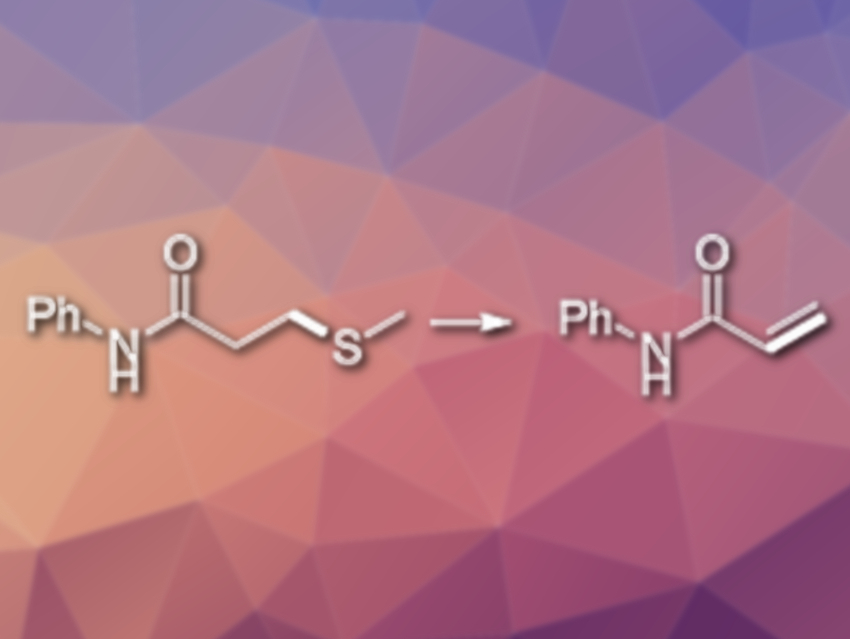
Ke Yang, Yi Li, Zhiyan Ma, Long Tang, Yue Yin, Hao Zhang, Zhengyi Li and Xiaoqiang Sun, Changzhou University, China, have developed an efficient metal-free C−S bond cleavage process. The method allows the team to access different β-aminopropanamide and acrylamide derivatives. Using Selectfluor as an “F+” reagent, a variety of N-substituted acrylamides were synthesized from N-substituted 3-methylthiopropanamides in 1,4‐dioxane (reaction pictured). The products were obtained in good to excellent yields.
The reaction can also be used to convert the thioethers to amine derivatives: When different N-substituted 3-methylthiopropanamides were reacted with amines in the presence of Selectfluor, N-substituted β-aminopropanamides were obtained in moderate to good yields.
Mechanistic investigations indicate that the N-substituted 3-methylthiopropanamides are converted the corresponding thionium intermediates or sulfoxides in the presence of Selectfluor, both of which can then be further transformed into N-substituted acrylamides and β-aminopropanamides.
- Metal-Free C–S Bond Cleavage to Access N-Substituted Acrylamide and β-Aminopropanamide,
Ke Yang, Yi Li, Zhiyan Ma, Long Tang, Yue Yin, Hao Zhang, Zhengyi Li, Xiaoqiang Sun,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900960



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)