Energy-Efficient Solar Photochemistry
The sun could be used as a sustainable energy source to power photochemical reactions. Timothy Noël, Eindhoven University of Technology, The Netherlands, and colleagues have developed a widely applicable, cost-effective photomicroreactor. It is based on “luminescent solar concentrators”, which harvest and convert photons and make them available for chemical reactions. Thus, the researchers were able to synthesize various substances, including two pharmaceuticals.
To date, research into the use of sunlight has focused on solar electricity, solar thermal, and solar fuels, while the solar-powered synthesis of chemicals is still in its infancy. Light energy can power chemical reactions, for example, by moving a catalyst into an excited state and thereby accelerating a reaction or even making it possible in the first place. However, the sun as a light source is disadvantageous in certain respects because the bulk of the solar spectral irradiance (the radiant flux received by a surface per unit area) falls within the relatively narrow visible range. Moreover, fluctuations in irradiance are caused by phenomena such as passing clouds.
The team has shown for the first time that a diverse set of photon-driven transformations can be efficiently powered by solar irradiation. The secret of success is their specially designed, cost-effective “photomicroreactor” based on luminescent solar concentrators (LSCs).
Luminescent Solar Concentrators
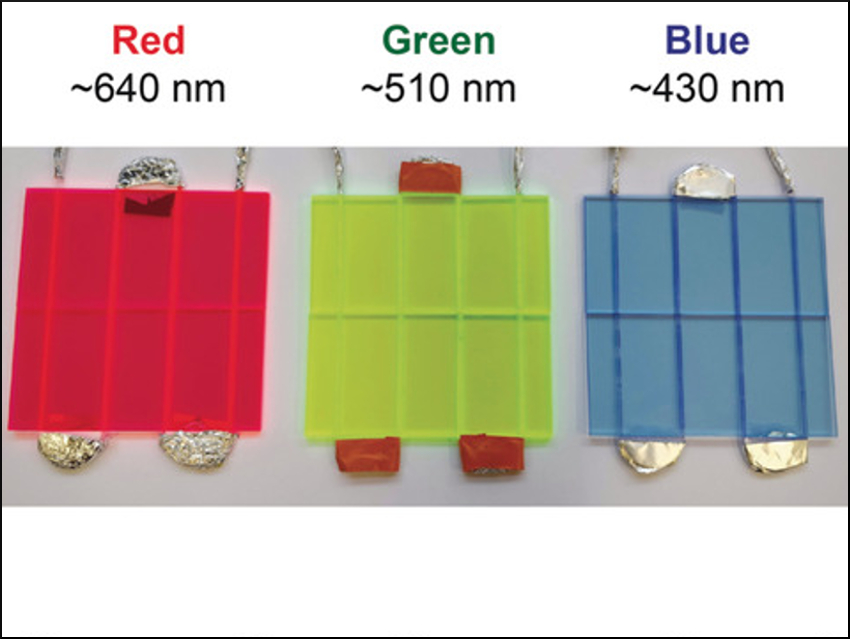
The LSCs consist of light-guiding slabs made of polymethylmethacrylate (PMMA) doped with special luminophores that capture photons from the solar spectrum and subsequently release them as fluorescence with longer wavelength characteristics for use by the luminophore. In this way, the sunlight is concentrated into a narrow wavelength range, and daylight and weather-dependent fluctuations of the spectral distribution become negligible.
Tiny channels made of a solvent-resistant polymer which contain the reaction mixture are embedded into the LSC slabs. A light sensor that monitors the light intensity is connected to an integrated circuit that autonomously adjusts the flow rate of the mixture: the lower the light intensity, the slower the mixture passes the channel, thereby receiving the light dose needed for an adequate reaction yield. In so doing, fluctuations in sunlight irradiance are compensated for and the quality of the product remains consistent.
The selection of the doping luminophores depends on the wavelength needed for excitation of the catalyst. The team generated red, green, and blue LSC reactors for reactions catalyzed by the photocatalysts methylene blue for the red device, eosin Y and rose Bengal for the green, and ruthenium-based metal complexes for the blue reactor. “Using these devices, we succeeded in synthesizing the antiworm drug ascaridole and an intermediate of the antimalarial drug artemisinin, besides others,” says Noël. “A solar-based production approach is of high interest for products with high added value, such as fine chemicals, drugs, and fragrances. It would be particularly suited to limited-resource settings.”
- Energy-Efficient Solar Photochemistry with Luminescent Solar Concentrator Based Photomicroreactors,
Dario Cambié, Jeroen Dobbelaar, Paola Riente, Jochen Vanderspikken, Chong Shen, Peter H. Seeberger, Kerry Gilmore, Michael G. Debije, Timothy Noël,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201908553