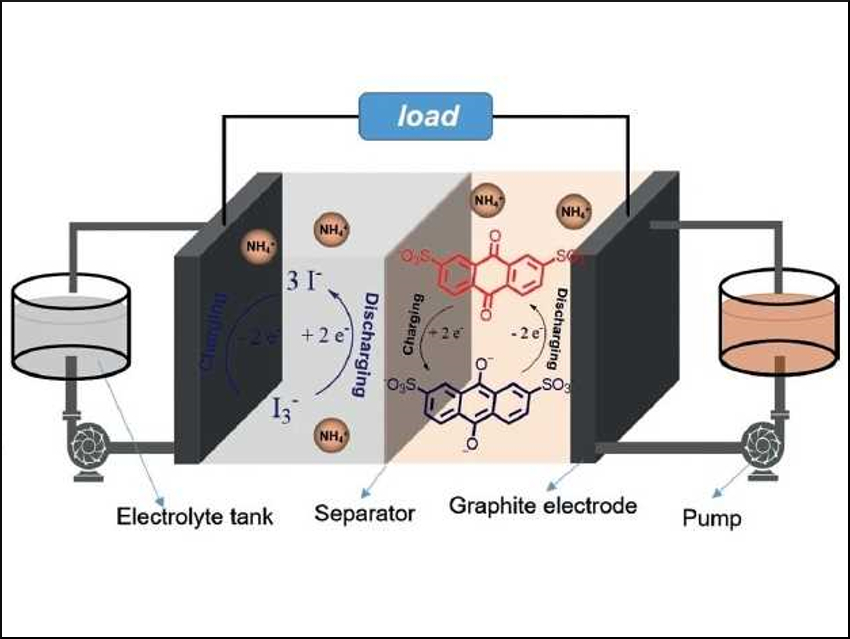
The sodium salt of anthraquinone-2,7-disulfonic acid (AQDS), AQDSNa2, is a highly stable, commercially available redox-active material. However, it has a low solubility in water. This must be improved before the salt can be used in the anolyte (i.e., the electrolyte around the anode) of aqueous organic redox flow batteries.
T. Leo Liu, Utah State University, Logan, USA, and colleagues have developed a strategy to solve this solubility issue via a cation exchange from Na+ to the more hydrophilic NH4+. The ammonium salt of AQDS was synthesized on a 20 g scale. The solubility of AQDS(NH4)2 in water is more than three times higher than for AQDSNa2. AQDS(NH4)2 was characterized by NMR spectroscopy, elemental analysis, and electrochemical studies. The NMR studies show that hydrogen bonding between the AQDS anions and the ammonium cations enhances the solvation of AQDS anions and, thus, contributes to the increased solubility.
A battery with 0.75 M AQDS(NH4)2 (pictured), paired with an ammonium iodide catholyte, delivered a high capacity up to 40.2 A h/L when operated under pH-neutral conditions. It was stable even after 300 charge/discharge cycles. These results provide a general, simple strategy to improve the solubility of active materials for applications in redox flow batteries.
- A pH-Neutral, Metal-Free Aqueous Organic Redox Flow Battery Employing an Ammonium Anthraquinone Anolyte,
Bo Hu, Jian Luo, Maowei Hu, Bing Yuan, T. Leo Liu,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201907934