The configuration of C=C double bonds affects the physical, chemical, and biological properties of alkenes significantly. Hence, methods for the synthesis of stereochemically defined functionalized alkenes, especially for the thermodynamically less stable Z-alkenes, are important.
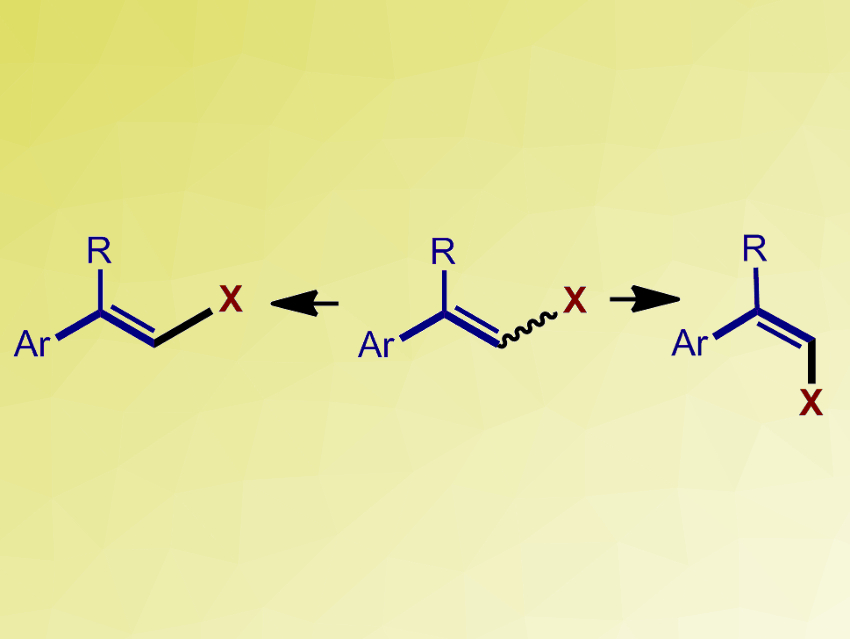
Qing Xu, Lei Yu, Yangzhou University, Jiangsu, China, Shouyun Yu, Nanjing University, China, and colleagues have developed an efficient and general method for the photocatalytic isomerization of styrenyl halides. Both (E)- and (Z)-isomers can be obtained with high stereoselectivity from E/Z-mixtures of styrenyl halides (pictured). The team used fac-Ir(ppy)3 (ppy = 2-phenylpyridine) as a photocatalyst for the E to Z isomerization (in methanol) and fluorescein as a photocatalyst for the Z to E isomerization (in 1,4-dioxane). Both reactions were performed under blue light-emitting diodes (LEDs) at room temperature under a nitrogen atmosphere.
The resulting styrenyl halides can be used in subsequent cross-coupling reactions, which can be run as a one-pot transformation together with the isomerization. The method can be used for the stereoselective synthesis of biologically important alkenes, for example, the antitumor drug DMU-212 and its Z-isomer.
- Photocatalytic Isomerization of Styrenyl Halides: Stereodivergent Synthesis of Functionalized Alkenes,
Hao Zhang, Qing Xu, Lei Yu, Shouyun Yu,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901119