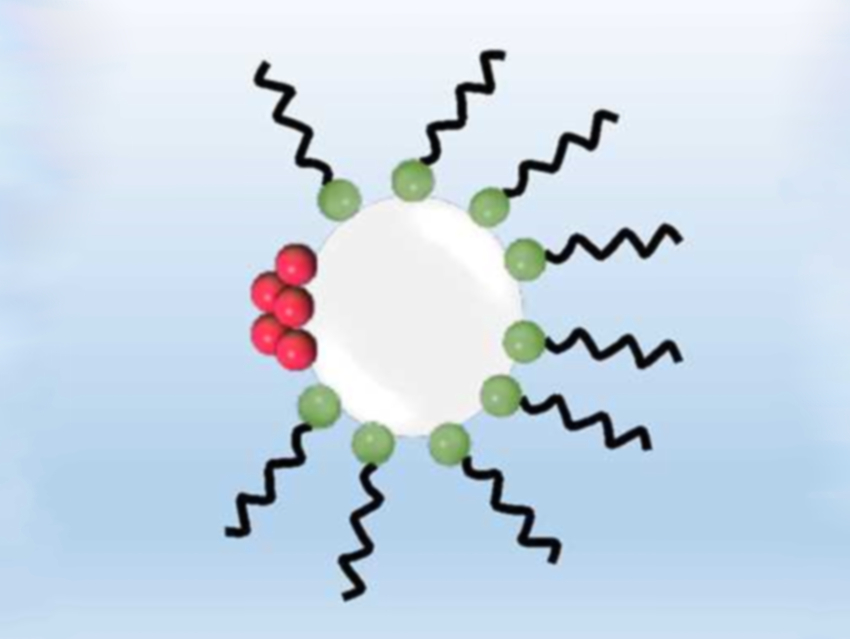
The crystallization process in microreactors can be enhanced by continuously introducing microbubbles. They provide a heterogeneous surface for nucleation, resulting in larger crystals and earlier nucleation. Additionally, they also prevent clogging of the reactor.
In static environments, the addition of surfactants such as sodium dodecyl sulfate (SDS) increases crystallization. In a continuous process, the addition of a surfactant is expected to create smaller microbubbles by decreasing the gas-liquid interfacial tension. The bubbles should also appear faster and more frequently, thereby increasing the bubble surface area per reactor volume.
Based on these assumptions, Simon Kuhn, KU Leuven, Belgium, and colleagues tested if the addition of SDS (pictured in green and black) can enhance the crystallization of paracetamol (pictured in red) in a continuous flow microreactor. As expected, the bubble diameter was reduced by half, and the bubble generation was five times faster than without SDS. Due to the reduced surface tension, the bubbles break off earlier. The uneven distribution of the SDS on the phase interface also leads to slower velocity. This confirms that the addition of SDS increases the surface area per reactor volume and, thereby, the number of possible nucleation sites.
However, crystal growth experiments showed that SDS prevents these sites from starting nucleation and that it takes longer for crystals to form. Overall, the increased bubble surface area cannot counteract the suppressed nucleation induced by SDS. The higher the SDS concentration, the more pronounced is this effect.
- Effect of Sodium Dodecyl Sulfate on the Continuous Crystallization in Microfluidic Devices Using Microbubbles,
Naghmeh Fatemi, Cedric Devos, Glenn Cordt, Tom Gerven, Simon Kuhn,
Chem. Eng. Technol. 2019, 42, 2105–2112.
https://doi.org/10.1002/ceat.201900172The article is part of a Chemical Engineering & Technology Special Issue on Micro Reaction Technology.