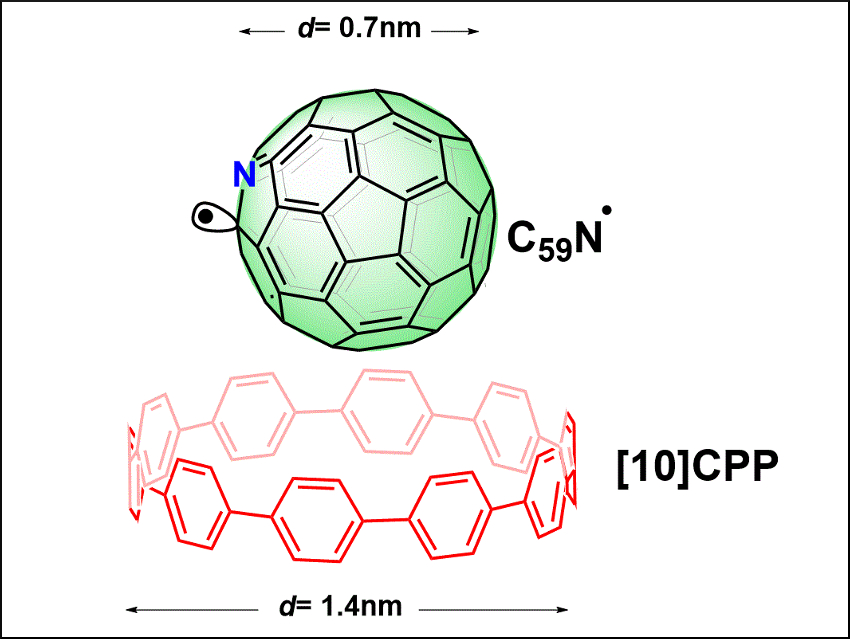
Unpaired electrons residing on fullerenes are interesting, e.g., for spintronics and energy-related applications. However, materials carrying free electrons are typically reactive and short-lived. A protective environment is required for shielding and assembling stable fullerene radicals.
Denis Arčon, University of Ljubljana, Slovenia, Hermann A. Wegner, University of Gießen, Germany, Christopher P. Ewels, Institut des Materiaux Jean Rouxel, Nantes, France, Nikos Tagmatarchis, National Hellenic Research Foundation, Athens, Greece, and colleagues have used a supramolecular design to protect the paramagnetic azafullerenyl C59N• radical (pictured in green): A ring-shaped [10]cycloparaphenylene molecule ([10]CPP, pictured in red) can encircle a stable dimer bisazafullerene (C59N)2 via concave-convex π-π interactions, and the fullerene–fullerene bond can then be broken by laser irradiation.
The generation and lifetime of the resulting paramagnetic C59N• were monitored by electron paramagnetic resonance (EPR) spectroscopy. Without [10]CPP, the C59N• radical has a sub-second lifetime. With [10]CPP, the characteristic C59N• triplet signal was observable even after several weeks. [10]CPP effectively blocks the recombination process and increases the lifetime of the radical by a factor of over 108.
According to the researchers, their radical shielding approach based on supramolecular complexation could pave the way for the use of fullerene radicals.
- A Long-Lived Azafullerenyl Radical Stabilized by Supramolecular Shielding with a [10]Cycloparaphenylene,
Anastasios Stergiou, Jérémy Rio, Jan H. Griwatz, Denis Arčon, Hermann A. Wegner, Christopher P. Ewels, Nikos Tagmatarchis,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201909126