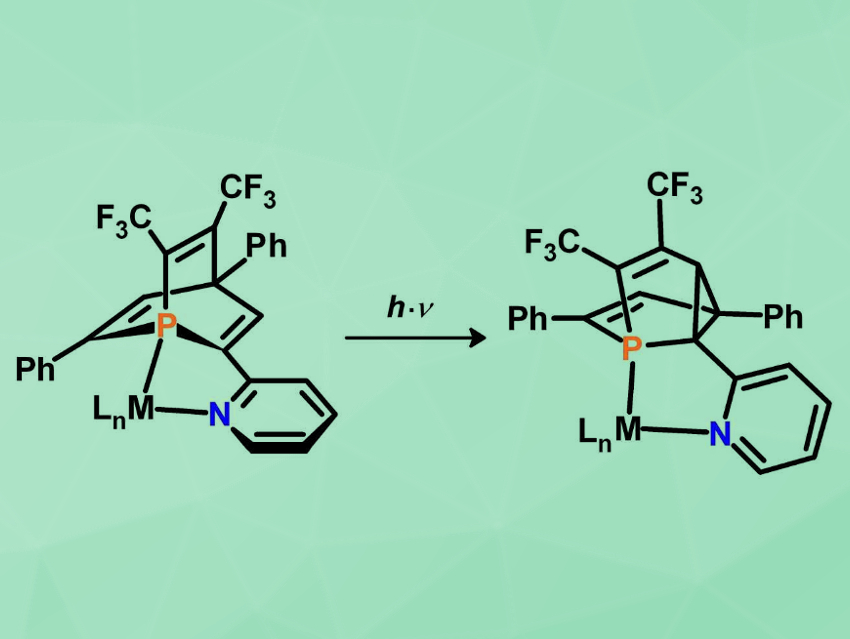
The di-π-methane rearrangement is a well-known photochemical reaction in organic chemistry. It can, for example, convert barrelene (C8H8) to its isomer semibullvalene. This reaction is generally possible for compounds consisting of two vinyl moieties connected to an sp3-hybridized carbon atom. Many examples have been reported. Variations with heteroatoms are also known, such as the oxa-di-π-methane and the aza-di-π-methane rearrangement.
Christian Müller, Freie Universität Berlin, Germany, and colleagues have recently discovered that 1-phosphabarrelenes, in which the central CH-group of the barrelene is replaced by an isolobal, trivalent phosphorus atom, can undergo a photochemical rearrangement to 5-phosphasemibullvalenes. This observation expanded the set of established di-π-methane rearrangements to unsaturated organophosphorus compounds.
As a next step in the chemistry of these phosphabarrelenes, the team has demonstrated that the photochemical di-π-methane rearrangement of a new pyridyl-functionalized and bis-CF3-substituted 1-phosphabarrelene (pictured left) occurs selectively in the coordination sphere of a metal center (LnM = RH(CO)Cl or W(CO)4). This led to the first transition-metal complexes containing chelating and chiral 5-phosphasemibullvalene derivatives (pictured right). Such coordination compounds could play an interesting role in transition-metal-mediated transformations and homogeneous catalysis.
- Pyridyl‐Functionalized 1‐Phosphabarrelene: Synthesis, Coordination Chemistry and Photochemical di‐π‐Methane Rearrangement,
Marlene Bruce, Martin Papke, Andreas W. Ehlers, Manuela Weber, Dieter Lentz, Nicolas Mézailles, J. Chris Slootweg, Christian Müller,
Chem. Eur. J.2019.
https://doi.org/10.1002/chem.201903344