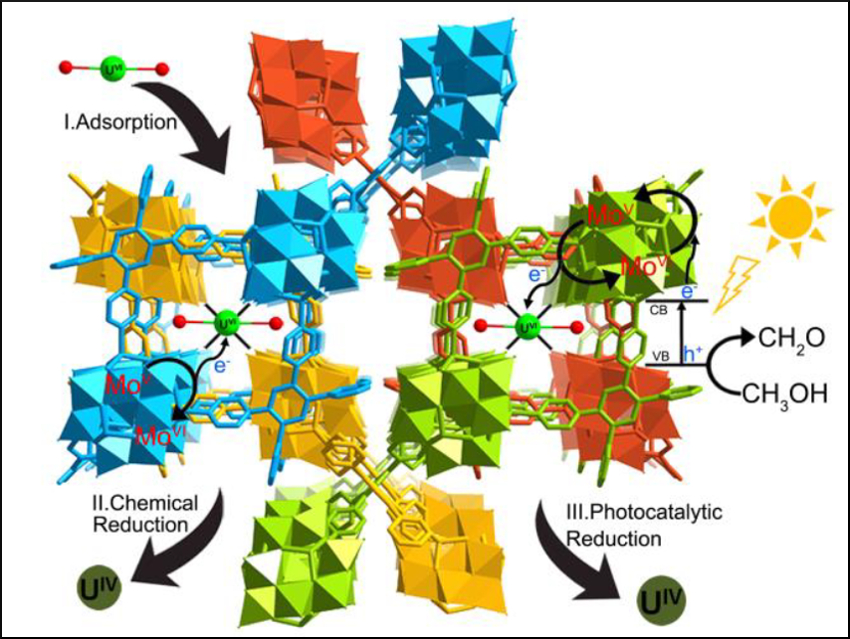
Uranium is a key resource in the nuclear fuel cycle. However, it is an environmental contaminant, both radioactive and toxic. Efficient and affordable uranium capture technologies are needed to reduce the costs of nuclear power and to simplify decontamination.
Shuao Wang, Soochow University, Suzhou, China, and colleagues, have synthesized a polyoxometalate (POM)–organic framework (SCU-19) using a solvothermal method. The framework has the composition [ϵ‐PMoV8MoVI4O37(OH)3Zn4](TPB)3/2⋅6 H2O (TPB = 1,2,4,5‐tetra(4‐pyridyl)benzene). SCU-19 was tested for uranium capture in darkness and under visible light. The mechanisms for uranium uptake were probed with elemental mapping, X-ray photoelectron (XPS) spectroscopy, and X-ray absorption near-edge (XANES) spectroscopy.
The team found that exposed oxo groups allow SCU-19 to complex and capture uranium efficiently. In darkness, low valent molybdenum atoms in the POM reduce UVI to a UIV species with reduced solubility and mobility. Under visible light, SCU-19 behaves like an n-type semiconductor and photocatalytically reduces UVI. The team believes that SCU-19 is the first uranium sorbent material with three distinct sorption mechanisms, and they suggest that the material could also be used to separate other radionuclides.
- Three Mechanisms in One Material: Uranium Capture by a Polyoxometalate-Organic Framework through Combined Complexation, Chemical Reduction, and Photocatalytic Reduction,
Hailong Zhang, Wei Liu, Ao Li, Duo Zhang, Xiaoyan Li, Fuwan Zhai, Lanhua Chen, Long Chen, Yanlong Wang, Shuao Wang,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201909718