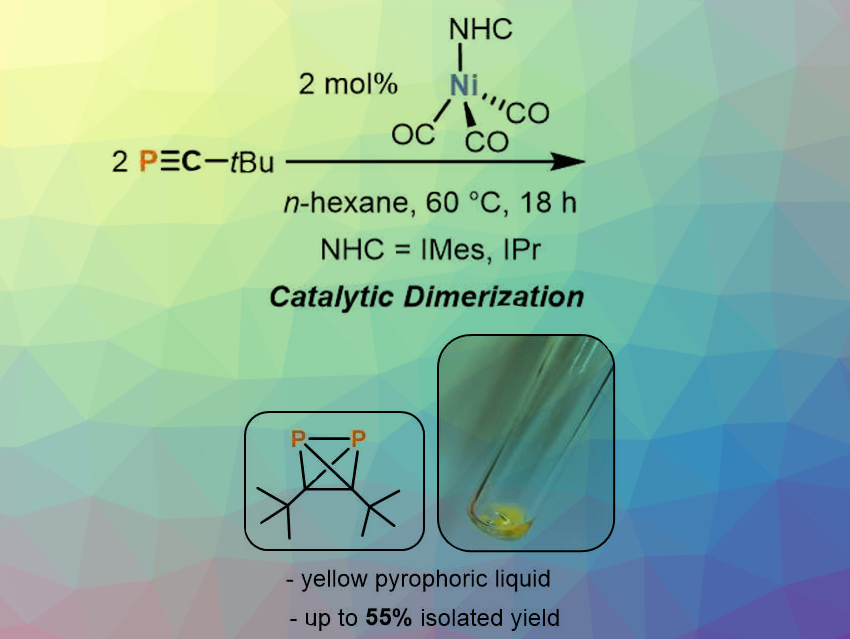
Tetrahedranes are highly strained and reactive molecules with a tetrahedral core structure. Neutral tetrahedranes containing two different atoms in the core are scarce, with AsP3 being the only example isolated so far. Tetrahedranes composed of carbon and phosphorus are particularly attractive research targets considering the diagonal relationship between carbon and phosphorus in the Periodic Table and the well-developed chemistry of white phosphorus (P4).
Robert Wolf and colleagues, University of Regensburg, Germany, have synthesized di-tert-butyldiphosphatetrahedrane (PCtBu)2, the first neutral tetrahedrane that contains carbon and phosphorus atoms in its core. Di‐tert‐butyldiphosphatetrahedrane, (tBuCP)2, is formed from the monomer tBuCP in a nickel‐catalyzed dimerization reaction using [(NHC)Ni(CO)3] (NHC = 1,3‐bis(2,4,6‐trimethylphenyl)imidazolin‐2‐ylidene (IMes) and 1,3‐bis(2,6‐diisopropylphenyl)imidazolin‐2‐ylidene (IPr)) (pictured above).
The molecule is stable enough to be isolated and thoroughly characterized. Complexation of (PCtBu)2 to AgI and single crystal X-ray anaysis of the resulting complex clearly showed the intact P2C2-tetrahedron coordinated to AgI. With the isolation of di-tert-butyldiphosphatetrahedrane, the team managed to resolve the structural identity of a free phosphaalkyne dimer. The present study closes a significant gap in the chemistry of phosphaalkynes.
- Di-tert -butyldiphosphatetrahedrane: Catalytic Synthesis of the Elusive Phosphaalkyne Dimer,
Gabriele Hierlmeier, Peter Coburger, Michael Bodensteiner, Robert Wolf,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201910505